Angiogenesis plays an important role in several physiological and pathological processes. Pharmacological angiogenesis modulation has been robustly demonstrated to achieve clinical benefits in several cancers. Adrenocortical carcinomas (ACC) are rare tumors that often have a poor prognosis. In addition, therapeutic options for ACC are limited. Understanding the mechanisms that regulate adrenocortical angiogenesis along the embryonic development and in ACC could provide important clues on how these processes could be pharmacologically modulated for ACC treatment. In this report, we performed an integrative review on adrenal cortex angiogenesis regulation in physiological conditions and ACC. During embryonic development, adrenal angiogenesis is regulated by both VEGF and Ang-Tie signaling pathways. In ACC, early research efforts were focused on VEGF signaling and this pathway was identified as a good prognostic factor and thus a promising therapeutic target.
- angiogenesis
- adrenal fetal cortex
- adrenocortical carcinoma
1. Introduction
Angiogenesis is a dynamic process during which new blood vessels are formed derived from pre-existing vasculature. Angiogenesis is an extensively studied process in tumors and a well-recognized hallmark of cancer [1]. Angiogenesis was previously studied in adrenocortical carcinomas (ACC), although the relative rarity of these tumors represents a limitation to conduct extensive clinical and molecular characterization studies. This review aims to bring together all the available data on angiogenesis regulation during the adrenocortical development and in ACC, which could be potentially useful to identify future research avenues to achieve advances in ACC clinical management and disease prognosis. Data source and study selection approach is described in the Supplementary File S1.
2. Angiogenesis Regulation
Angiogenesis plays a central role in several physiological (e.g., fetal development and wound healing) and pathological processes (e.g., vascular overgrowth for tumor expansion and metastasis) [2][3][4]. Angiogenesis, either in normal or tumor tissues, usually occurs via one or more of the following mechanisms:
- (1)
-
Sprouting angiogenesis, one the most well characterized mechanism leading to angiogenesis, relies on endothelial cells function specification into either tip or stalk cells. Tip cells are derived from the parent vessel, degrade the basement membrane, extend large filopodia which can sense angiogenic factor gradients, such as vascular endothelial growth factor (VEGF), and migrate along the chemotactic paths. In contrast, stalk cells proliferate behind tip cells to form the sprout body, start the process of lumen formation, and connect with neighboring vessels [5][6][7].
- (2)
-
Intussusceptive angiogenesis is a process that consists in the splitting of pre-existing vessels into two new vessels. It starts with the formation of transluminal tissue pillars through the invagination of opposing capillary endothelial cells into the vascular lumen, creating a zone of contact. Commonly, intussusceptive and sprouting angiogenesis are complementary mechanisms [5][8].
- (3)
-
Recruitment of endothelial progenitor cells and vasculogenesis, a process through which endothelial progenitor cells are recruited in response to several growth factors, cytokines and/or hypoxia-inducible factors. Endothelial progenitor cells differentiate into mature endothelial cells and are incorporated into the angiogenic sprout, thus contributing to new blood vessel formation [4][9].
- (4)
Multiple signaling pathways regulate blood vessel growth and maintenance. Among these, VEGF and Ang-Tie pathways are particularly important and have been the focus of multiple studies, especially in the context of cancer [12]. VEGF receptor and Tie ligands are widely distributed and were shown to play a coordinated role in endothelial cell proliferation and vessel wall assembly in normal and pathological conditions.
2.1. VEGF Pathway in Angiogenesis Regulation
In mammals, the VEGF system mainly includes five secreted ligands (VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor) and three primary tyrosine kinase receptors (VEGF-R1, VEGF-R2, VEGF-R3) [13]. The VEGF system also includes the cell-surface proteins, heparan sulfate proteoglycans and neuropilin-1 and -2, which operate as VEGF coreceptors [14][15].
VEGFR-1 and VEGFR-2 are expressed in vascular endothelial cells, while VEGF-R3 seems to be prominently expressed in lymphatic endothelial cells [16]. VEGF ligands have different affinities for one of the three VEGF-R. As tyrosine kinases receptors, upon dimerization by a VEFG ligand, the VEGF-Rs auto-phosphorylate, a phenomenon which in turn activates downstream signaling pathways including mitogen-activated protein kinase (MAPK) pathway, the phosphatidylinositol-3 kinase (PI3K-AKT) pathway, and the phospholipase-C-γ pathway. Those pathways drive various intracellular effects in endothelial cells, such as migration, proliferation, and cell survival. The activation of phospholipase-C-γ pathway via VEGF-A-VEGFR-2 binding was reported to be a key signal for endothelial proliferation [17].
In 2001, a new VEGF was identified, the endocrine-gland-derived VEGF (EG-VEGF). This ligand does not show any structural homology to the VEGF family, but displays several biological similarities to VEGF ligands, including hypoxic regulation and ability to induce fenestration in target cells. Moreover, EG-VEGF expression is restricted to steroidogenic tissues (adrenal, ovary, testis and placenta) and its effects seem to be restricted to endothelial cells derived from these organs [18].
2.2. Ang-Tie Pathway in Angiogenesis Regulation
Ang-Tie signaling pathway regulates vascular permeability and remodeling during tumor angiogenesis and metastasis. Ang/Tie signaling seems to complement the VEGF signaling pathway by controlling later stages of angiogenesis and by being involved in vascular maturation (Figure 1) [19].
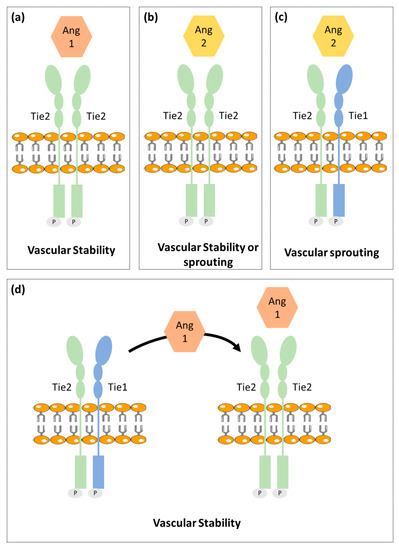
Figure 1. Schematic illustration of the vessel stability regulation by Ang/Tie signaling. (a) Ang1 binds to Tie2 promoting vascular stability. (b) Ang2 has a dual function acting as a Tie2 agonist or antagonist to promote vascular stability or sprouting, respectively. (c) Tie1 forms a complex with Tie2. Upon Ang2 stimulation, Tie1 and Tie2 remain associated and Ang2 induces vascular sprouting. (d) Ang1 stimulation promotes Tie2 clustering leading to vascular stability. This schematic representation includes the most consensual theories; however, this pathway is not yet fully understood. Besides that, this figure is a schematic representation that is not intended to translate the real chemical conformation of the proteins.
The angiopoietin family includes two type 1 transmembrane protein receptors: Tie1 and Tie2 and four ligands: Ang1, Ang2, Ang3 and Ang4. Ang1 and Ang2 have been identified as the main ligands for Tie receptors, while the Ang3 and Ang4 biological function is still poorly characterized [20][21][22].
Ang1 binds and activates Tie2 resulting in Tie2 internalization and ligand release. Then it leads to Tie2 tyrosine residues phosphorylation that in turn recruits adaptor proteins and ignites PI3K/Akt and MAPK signaling pathways, promoting pro-survival, anti-permeability, and anti-inflammatory effects on endothelial cells [23]. Tie2 is not required for the endothelial cells’ differentiation but is rather reported as necessary for cell maintenance [24].
Ang2 that shares approx. 60% amino acid homology with Ang1, binds to Tie2 with a similar affinity as Ang1. Ang2 seems to block the Ang1-induced Tie2 phosphorylation. Ang2 is upregulated during tumor angiogenesis and so was considered as a potential antiangiogenic target. However, recent studies found Ang2 to have a dual function, acting as a Tie2 antagonist in the presence of Ang1 or acting as a Tie2 agonist in the absence of Ang1 [25]. Different studies reported that it is unlikely that Tie2 can act differently when binding to Ang1 and Ang2, since both angiopoietins interact with Tie2 in a structurally similar manner and pointed out that other still unidentified mechanisms were likely to be involved [26]. One of the proposed mechanisms involve the Tie1 receptor [27].
Contrary to Tie2, the Tie1 has been less well characterized. Tie1 is considered an orphan receptor and is mainly expressed at vascular bifurcations and branching points, with no yet identified in vivo ligand [28]. It is well known, however, that Tie1 has an important role in vascular development, since its inactivation causes late embryonic lethality and vasculature maturation failure [29][30]. Recent studies proposed that Tie1 forms a complex with Tie2 on the endothelial cell surface and acts as a Tie2 inhibitor [27]. Cells expressing both receptors are responsive to chemotactic signals and able to promote vessel branching and sprouting that is required for angiogenesis. On the other hand, Tie1 is absent in stable and quiescent mature vessels [27].
A mechanistic study indicated Tie1 as being responsible for angiopoientin’s differential function. In mature vessels, as Tie1 is absent, Tie2 can be activated by either Ang1 or Ang2, to promote vessel stability. On active angiogenesis sites, Tie1 and Tie2 form a complex and Ang2 fails to activate Tie2, allowing vessel branching to be promoted. On the other hand, Ang1 is able to dissociate Tie2 from the Tie1-Tie2 complex, activating Tie2 and thus enhancing vascular stability [27][31].
3. Angiogenesis in Normal Adrenal Cortex
3.1. Fetal Adrenal Cortex
Human fetal adrenal (HFA) plays a critical role in fetal maturation and perinatal survival. HFA steroid hormones regulate intrauterine homeostasis and appropriate fetal organ systems maturation [32][33].
Contrary to the adult adrenal cortex that includes three distinct zones: glomerulosa, fasciculata and reticularis; the HFA is primarily composed of two single distinct zones: outer zone or definitive zone and inner zone or fetal zone [33][34]. The definitive zone comprises a narrow band of small cells that exhibit typical characteristics of cells in proliferative state. Definitive zone does not produce steroids until the third trimester. However, as gestation advances, definitive zone cells start to accumulate lipids and resemble steroidogenic active cells. The fetal zone is the largest adrenal cortex zone and consists of large cells that exhibit features characteristic of steroid-secreting cells [33][34][35][36][37]. In ultrastructural studies, a third zone in between definitive zone and fetal zone, named transitional zone, has been described. The transitional zone is composed by cells with intermediate characteristics, but capable to synthetize cortisol and so cells can be considered analogous to fasciculata layer cells of mature adrenal cortex [33][38][39][40][41].
Due to the HFA critical role in fetal maturation, the early and extensive vasculature development that occurs in this gland, is not only necessary but also particularly important. Angiogenesis is not only required for HFA growth and maturation, but it is also necessary for the influx of steroid precursors and trophic factors into the gland to enable mature steroids synthesis and secretion into circulation. Indeed, the fetal adrenal gland is one of the most highly vascularized organs in the human fetus [41].
Previous studies have reported that VEGF-A, FGF-2, Ang1, Ang2, and Tie2 are expressed in HFA since midgestation and to have a putative role in adrenal gland angiogenesis [34][42][43].
Ang2 expression in HFA is markedly higher when compared to the mature adrenal gland, whereas Ang1 and Tie2 expression seem to be similar in both fetal and adult adrenals. Thus, supporting higher angiogenesis activity and vascular instability in developing adrenal glands [42].
Ang2, FGF-2 and VEGF-A expression are mainly expressed in the gland periphery suggesting that the HFA periphery is the primary site of angiogenesis, in parallel to cell proliferation [42][43]. Further supporting this hypothesis, a dense network of irregular capillaries was also observed at the HFA periphery [44].
On the contrary, Ang1 is mainly expressed in the fetal zone, suggesting that the inner adrenal zone presents a greater vessel maturity. Tie2, was exclusively identified to be present in endothelial cells throughout the gland [42][43].
Adrenocorticotropic hormone (ACTH), the main regulator of HFA growth and function, also seems to be implicated in angiogenesis control. In vitro studies found that ACTH upregulates VEGF-A, FGF-2 and Ang2 in the HFA, therefore controlling angiogenesis while simultaneously exerting growth and secretion stimulatory actions [42][43][45][46].
The steroidogenic factor 1 has a critical role in adrenal development, steroidogenesis, and also in gonadal differentiation [47]. In addition, steroidogenic factor 1 also seems to be implicated in HFA angiogenesis regulation by direct interaction and activation of the Ang2 gene promoter. Furthermore, the authors demonstrated that steroidogenic factor 1 and Ang2 are strongly co-expressed in HFA periphery in early stages of development [48].
Overall, these findings support that the adrenal gland growth, steroidogenesis and blood vessel formation, are synchronized phenomena [42][43][45][46].
3.2. Adult Adrenal Cortex
The adrenal gland is one of the most vascularized organs in adult mammalian organisms. Its developed intrinsic vasculature is required for an efficient secretion of steroid hormones into the systemic blood flow. The adrenal gland is supplied by three different arterial branches derived the abdominal aorta: inferior phrenic artery, middle adrenal artery and renal artery. The arterial blood enters in the adrenal gland and flows centripetally through the adrenal cortex into the adrenal medulla [49][50].
Previous studies have found that adrenocortical cells highly express VEGF-A and EG-VEGF—a VEGF specific of steroidogenic organs, both having been pointed out as important molecules for maintenance of the dense and fenestrated vasculature of the adrenal cortex. This expression also seems to be regulated by ACTH [51][52][53][54][55].
In addition, the vasculature of the adrenal cortex seems to be coordinated with the mass of the adrenal cortex, since it suffers fluctuations decreasing or increasing along regression or expansion of the adrenal cortex, respectively [52].
4. Angiogenesis in Adrenocortical Tumors
Adrenocortical tumors (ACT) are common adrenal tumors affecting 3% to 10% of the human population [56]. The majority of ACT are benign non-functioning adrenocortical adenomas (ACA), while malignant ACC are rare with an incidence of 0.7 to 2 per million per year [56]. ACC most often have a poor prognosis and are frequently already metastasized when first diagnosed. ACC pathogenesis is still largely unclear, which results in a lack of biomarkers available for diagnosis and in limited treatment options [57][58].
The status of the VEGF pathway in adrenocortical tumors has been already addressed in multiple studies (Table 1).
Table 1. VEGF pathway findings in adrenocortical tumors.
| Patient Group Comparisons | Results | |
|---|---|---|
| VEGF | Patients with ACT vs. Healthy individuals | ↑ VEGF serum levels in patients with ACT [59][60] |
| Aldosterone secreting ACA vs. Non-functioning ACA | ↑ VEGF tumor expression in aldosterone producing ACA [61] | |
| Cortisol secreting ACA vs. Aldosterone secreting ACA | ↑ VEGF serum levels patients with cortisol secreting ACA [60] | |
| ACC vs. Normal adrenal glands | ↑ VEGF expression in ACC [61][62] |
|
| ACC vs. ACA | ↑ VEGF serum levels in ACC ↑ VEGF tumor expression in ACC [59][61][63][64] |
|
| Patients with recurrent ACC vs. Patients with non-recurrent ACC | ↑ VEGF serum levels in recurrent ACC ↑ VEGF tumor expression in recurrent ACC [60][63] |
|
| Localized ACC vs. Invasive ACC | No difference in VEGF tumor expression [63] |
|
| VEGF-R2 | ACC vs. Normal adrenal glands | ↑ VEGF-R2 tumor expression in ACC [62] |
| ACC vs. ACA | ↑ VEGF-R2 tumor expression in ACC [64] |
ACA—Adrenocortical Adenomas; ACC—Adrenocortical carcinomas; ACT—Adrenocortical tumors; VEGF—Vascular endothelial growth factor; VEGFR—Vascular endothelial growth factor receptor; ↑—Increased protein levels or expression.
Patients with ACT were found to present higher VEGF serum levels as compared to healthy controls [59][60]. In addition, Kolomecki et al. demonstrated that VEGF serum levels were significantly higher in patients with non-functioning malignant tumors than in patients with non-functioning ACA. Noteworthy, VEGF serum levels in patients with ACC were shown to decrease after tumor surgical resection and increase in patients who experienced tumor recurrence [59]. de Fraipont et al. found that cytosolic VEGF-A concentrations were higher in ACC when compared to ACA, although not being significantly different when localized and more invasive ACC were compared [63]. Nevertheless, cytosolic VEGF-A concentrations were higher in recurrent as compared to non-recurrent ACC after primary tumor resection [63].
Tumor VEGF expression was also found to be higher in ACC as compared to normal adrenal glands and ACA [61][62][64]. VEGF receptor 2 tumor expression was also found to be higher in ACC when compared with ACA and normal adrenal glands [62][64].
Bernini et al., however, found that tumor VEGF expression was not directly related with vascular density, which was lower in ACC as compared to ACA and normal adrenal tissue. The fact that a higher VEGF expression was not shown to be associated with increased vascular density in ACC, was somehow unsurprising since a high vascular density already characterizes normal adrenal cortex tissue. What surprised researchers was that despite ACC lower vascular density, patients still had a very short survival time [61].
Other studies reported that although no differences in vascular density were noticed when ACC, ACA and normal adrenal glands were compared, blood vessels perimeter and area were higher in ACC when compared to ACA [65][66]. In addition, endothelial cell proliferation was higher in ACC [66].
On an opposed direction, another group reported vascular density to be higher in malignant ACT as compared to benign ACT [67]. Another study observed that in their series VEGF expression was positively correlated with vessel density [64]. Pereira et al. also reported ACC to present a higher vascular density, but only when compared to cortisol secreting ACA [68]. This could, however, be derived from cortisol anti-angiogenic effects [69]. There is additional evidence supporting that adrenocortical angiogenic status could be tightly related to the tumor’s hormonal functionality. Bernini et al. found that VEGF tumor expression was higher in aldosterone secreting ACA as compared to non-functioning ACA and normal adrenal glands [61]. In addition, in another study patients with cortisol-secreting ACA were found to have higher circulating VEGF levels than patients with aldosterone secreting adenomas [60].
The discovery of EG-VEGF, a steroidogenic organ specific VEGF, brought some enthusiasm to the scientific community as a potential explanation to the contradictory angiogenic patterns in ACTs as well as a potential target for ACC treatment. Heck et al. characterized the expression of EG-VEGF and its receptors [prokineticin receptor 1 (PKR1) and 2 (PKR2)] in a large number of ACC, ACA and normal adrenal glands. In this study, EG-VEGF and both receptors PKR1 and PKR2 were found to be present in the majority of ACT. Moreover, the nuclear protein expression of either EG-VEGF or PKR1 or both in ACC was reported to be associated with higher mortality, suggesting that these could be used as prognostic markers for overall patient survival [53].
New prognostic and diagnostic markers are needed to improve ACC clinical practice. As described in this section, the usefulness of angiogenic factors for ACC diagnosis and/or prognosis was already investigated. From those, VEGF was the one with more consistent and replicable results, being increased in ACC when compared with ACA [59][61][63][64], in particular in the recurrent malignant tumors [60][63]. However, due to the rarity of ACC, the number of patients included in each study is small. So, in the future, to validate this result, multi-center studies are needed to increase the samples/participants’ number and to uniformize the methodological approach to analyze the VEGF tumors expression in ACT. Stratified analysis according to tumors functionality are needed since in previous studies, it showed to influence VEGF levels.
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46.
- Chung, A.S.; Ferrara, N. Developmental and Pathological Angiogenesis. Annu. Rev. Cell Dev. Biol. 2011, 27, 563–584.
- Zuazo-Gaztelu, I.; Casanovas, O. Unraveling the Role of Angiogenesis in Cancer Ecosystems. Front. Oncol. 2018, 8, 248.
- Mentzer, S.J.; Konerding, M.A. Intussusceptive angiogenesis: Expansion and remodeling of microvascular networks. Angiogenesis 2014, 17, 499–509.
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2019.
- Duran, C.L.; Howell, D.W.; Dave, J.M.; Smith, R.L.; Torrie, M.E.; Essner, J.J.; Bayless, K.J. Molecular Regulation of Sprouting Angiogenesis. Compr. Physiol. 2017, 8, 153–235.
- Makanya, A.N.; Hlushchuk, R.; Djonov, V.G. Intussusceptive angiogenesis and its role in vascular morphogenesis, patterning, and remodeling. Angiogenesis 2009, 12, 113.
- Kolte, D.; McClung, J.A.; Aronow, W.S. Vasculogenesis and angiogenesis. In Translational Research in Coronary Artery Disease; Elsevier: Amsterdam, The Netherlands, 2016; pp. 49–65.
- Ge, H.; Luo, H. Overview of advances in vasculogenic mimicry—A potential target for tumor therapy. Cancer Manag. Res. 2018, 10, 2429–2437.
- Fernández-Cortés, M.; Delgado-Bellido, D.; Oliver, F.J. Vasculogenic Mimicry: Become an Endothelial Cell “But Not So Much”. Front. Oncol. 2019, 9.
- Saharinen, P.; Eklund, L.; Pulkki, K.; Bono, P.; Alitalo, K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol. Med. 2011, 17, 347–362.
- Takahashi, H.; Shibuya, M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. 2005, 109, 227–241.
- Guo, H.-F.; Vander Kooi, C.W. Neuropilin Functions as an Essential Cell Surface Receptor. J. Biol. Chem. 2015, 290, 29120–29126.
- Chiodelli, P.; Mitola, S.; Ravelli, C.; Oreste, P.; Rusnati, M.; Presta, M. Heparan sulfate proteoglycans mediate the angiogenic activity of the vascular endothelial growth factor receptor-2 agonist gremlin. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e116–e127.
- Cross, M.J.; Dixelius, J.; Matsumoto, T.; Claesson-Welsh, L. VEGF-receptor signal transduction. Trends Biochem. Sci. 2003, 28, 488–494.
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105.
- LeCouter, J.; Kowalski, J.; Foster, J.; Hass, P.; Zhang, Z.; Dillard-Telm, L.; Frantz, G.; Rangell, L.; DeGuzman, L.; Keller, G.A.; et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 2001, 412, 877–884.
- Augustin, H.G.; Koh, G.Y.; Thurston, G.; Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 2009, 10, 165–177.
- Lee, H.J.; Cho, C.H.; Hwang, S.J.; Choi, H.H.; Kim, K.T.; Ahn, S.Y.; Kim, J.H.; Oh, J.L.; Lee, G.M.; Koh, G.Y. Biological characterization of angiopoietin-3 and angiopoietin. FASEB J 2004, 18, 1200–1208.
- Eklund, L.; Saharinen, P. Angiopoietin signaling in the vasculature. Exp. Cell Res. 2013, 319, 1271–1280.
- Fagiani, E.; Christofori, G. Angiopoietins in angiogenesis. Cancer Lett. 2013, 328, 18–26.
- Davis, S.; Aldrich, T.H.; Jones, P.F.; Acheson, A.; Compton, D.L.; Jain, V.; Ryan, T.E.; Bruno, J.; Radziejewski, C.; Maisonpierre, P.C.; et al. Isolation of Angiopoietin-1, a Ligand for the TIE2 Receptor, by Secretion-Trap Expression Cloning. Cell 1996, 87, 1161–1169.
- Dumont, D.J.; Gradwohl, G.; Fong, G.H.; Puri, M.C.; Gertsenstein, M.; Auerbach, A.; Breitman, M.L. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994, 8, 1897–1909.
- Yuan, H.T.; Khankin, E.V.; Karumanchi, S.A.; Parikh, S.M. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol. Cell Biol. 2009, 29, 2011–2022.
- Barton, W.A.; Tzvetkova-Robev, D.; Miranda, E.P.; Kolev, M.V.; Rajashankar, K.R.; Himanen, J.P.; Nikolov, D.B. Crystal structures of the Tie2 receptor ectodomain and the angiopoietin-2-Tie2 complex. Nat. Struct. Mol. Biol. 2006, 13, 524–532.
- Seegar, T.C.M.; Eller, B.; Tzvetkova-Robev, D.; Kolev, M.V.; Henderson, S.C.; Nikolov, D.B.; Barton, W.A. Tie1-Tie2 interactions mediate functional differences between angiopoietin ligands. Mol. Cell 2010, 37, 643–655.
- Porat, R.M.; Grunewald, M.; Globerman, A.; Itin, A.; Barshtein, G.; Alhonen, L.; Alitalo, K.; Keshet, E. Specific Induction of tie1 Promoter by Disturbed Flow in Atherosclerosis-Prone Vascular Niches and Flow-Obstructing Pathologies. Circ. Res. 2004, 94, 394–401.
- Puri, M.C.; Rossant, J.; Alitalo, K.; Bernstein, A.; Partanen, J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 1995, 14, 5884–5891.
- La Porta, S.; Roth, L.; Singhal, M.; Mogler, C.; Spegg, C.; Schieb, B.; Qu, X.; Adams, R.H.; Baldwin, H.S.; Savant, S.; et al. Endothelial Tie1-mediated angiogenesis and vascular abnormalization promote tumor progression and metastasis. J. Clin. Investig. 2018, 128, 834–845.
- Zhang, Y.; Kontos, C.D.; Annex, B.H.; Popel, A.S. Angiopoietin-Tie Signaling Pathway in Endothelial Cells: A Computational Model. iScience 2019, 20, 497–511.
- Liggins, G.C. Adrenocortical-related maturational events in the fetus. Am. J. Obstet. Gynecol. 1976, 126, 931–941.
- Mesiano, S.; Jaffe, R.B. Developmental and functional biology of the primate fetal adrenal cortex. Endocr. Rev. 1997, 18, 378–403.
- Ishimoto, H.; Jaffe, R.B. Development and function of the human fetal adrenal cortex: A key component in the feto-placental unit. Endocr. Rev. 2011, 32, 317–355.
- Lanman, J.T. The fetal zone of the adrenal gland: Its developmental course, comparative anatomy, and possible physiologic functions. Medicine 1953, 32, 389–430.
- Johannisson, E. The foetal adrenal cortex in the human. Its ultrastructure at different stages of development and in different functional states. Acta Endocrinol. 1968, 58, S7–S107.
- Spencer, S.J.; Mesiano, S.; Lee, J.Y.; Jaffe, R.B. Proliferation and apoptosis in the human adrenal cortex during the fetal and perinatal periods: Implications for growth and remodeling. J. Clin. Endocrinol. Metab. 1999, 84, 1110–1115.
- McNutt, N.S.; Jones, A.L. Observations on the ultrastructure of cytodifferentiation in the human fetal adrenal cortex. Lab. Investig. J. Tech. Methods Pathol. 1970, 22, 513–527.
- Sucheston, M.E.; Cannon, M.S. Development of zonular patterns in the human adrenal gland. J. Morphol. 1968, 126, 477–491.
- Mesiano, S.; Coulter, C.L.; Jaffe, R.B. Localization of cytochrome P450 cholesterol side-chain cleavage, cytochrome P450 17 alpha-hydroxylase/17, 20-lyase, and 3 beta-hydroxysteroid dehydrogenase isomerase steroidogenic enzymes in human and rhesus monkey fetal adrenal glands: Reappraisal of functional zonation. J. Clin. Endocrinol. Metab. 1993, 77, 1184–1189.
- McClellan, M.C.; Brenner, R.M. Development of the fetal adrenals in nonhuman primates: Electron microscopy. In Fetal Endocrinology; Novy, M.J., Resko, J.A., Eds.; Academic Press: Cambridge, MA, USA, 1981; Volume 1, pp. 383–403.
- Ishimoto, H.; Ginzinger, D.G.; Jaffe, R.B. Adrenocorticotropin preferentially up-regulates angiopoietin 2 in the human fetal adrenal gland: Implications for coordinated adrenal organ growth and angiogenesis. J. Clin. Endocrinol. Metab. 2006, 91, 1909–1915.
- Ishimoto, H.; Minegishi, K.; Higuchi, T.; Furuya, M.; Asai, S.; Kim, S.H.; Tanaka, M.; Yoshimura, Y.; Jaffe, R.B. The periphery of the human fetal adrenal gland is a site of angiogenesis: Zonal differential expression and regulation of angiogenic factors. J. Clin. Endocrinol. Metab. 2008, 93, 2402–2408.
- Pitynski, K.; Litwin, J.A.; Nowogrodzka-Zagorska, M.; Miodonski, A.J. Vascular architecture of the human fetal adrenal gland: A SEM study of corrosion casts. Ann. Anat. Anat. Anz Off. Organ Anat. Ges. 1996, 178, 215–222.
- Mesiano, S.; Mellon, S.H.; Gospodarowicz, D.; Di Blasio, A.M.; Jaffe, R.B. Basic fibroblast growth factor expression is regulated by corticotropin in the human fetal adrenal: A model for adrenal growth regulation. Proc. Natl. Acad. Sci. USA 1991, 88, 5428–5432.
- Shifren, J.L.; Mesiano, S.; Taylor, R.N.; Ferrara, N.; Jaffe, R.B. Corticotropin regulates vascular endothelial growth factor expression in human fetal adrenal cortical cells. J. Clin. Endocrinol. Metab. 1998, 83, 1342–1347.
- Ozisik, G.; Achermann, J.C.; Meeks, J.J.; Jameson, J.L. SF1 in the development of the adrenal gland and gonads. Horm. Res. 2003, 59 (Suppl. 1), 94–98.
- Ferraz-de-Souza, B.; Lin, L.; Shah, S.; Jina, N.; Hubank, M.; Dattani, M.T.; Achermann, J.C. ChIP-on-chip analysis reveals angiopoietin 2 (Ang2, ANGPT2) as a novel target of steroidogenic factor-1 (SF-1, NR5A1) in the human adrenal gland. FASEB J. 2010, 25, 1166–1175.
- Sapirstein, L.A.; Goldman, H. Adrenal blood flow in the albino rat. Am. J. Physiol. 1959, 196, 159–162.
- Bassett, J.R.; West, S.H. Vascularization of the adrenal cortex: Its possible involvement in the regulation of steroid hormone release. Microsc. Res. Tech. 1997, 36, 546–557.
- Thomas, M.; Keramidas, M.; Monchaux, E.; Feige, J.J. Role of adrenocorticotropic hormone in the development and maintenance of the adrenal cortical vasculature. Microsc. Res. Tech. 2003, 61, 247–251.
- Vittet, D.; Ciais, D.; Keramidas, M.; De Fraipont, F.; Feige, J.J. Paracrine control of the adult adrenal cortex vasculature by vascular endothelial growth factor. Endocr. Res. 2000, 26, 843–852.
- Heck, D.; Wortmann, S.; Kraus, L.; Ronchi, C.L.; Sinnott, R.O.; Fassnacht, M.; Sbiera, S. Role of Endocrine Gland-Derived Vascular Endothelial Growth Factor (EG-VEGF) and Its Receptors in Adrenocortical Tumors. Horm. Cancer 2015, 6, 225–236.
- Senger, D.R. Vascular endothelial growth factor: Much more than an angiogenesis factor. Mol. Biol. Cell 2010, 21, 377–379.
- Gomez-Sanchez, C.E. Regulation of Adrenal Arterial Tone by Adrenocorticotropin: The Plot Thickens. Endocrinology 2007, 148, 3566–3568.
- Else, T.; Kim, A.C.; Sabolch, A.; Raymond, V.M.; Kandathil, A.; Caoili, E.M.; Jolly, S.; Miller, B.S.; Giordano, T.J.; Hammer, G.D. Adrenocortical Carcinoma. Endocr. Rev. 2014, 35, 282–326.
- Libé, R. Adrenocortical carcinoma (ACC): Diagnosis, prognosis, and treatment. Front. Cell Dev. Biol. 2015, 3, 45.
- Fassnacht, M.; Arlt, W.; Bancos, I.; Dralle, H.; Newell-Price, J.; Sahdev, A.; Tabarin, A.; Terzolo, M.; Tsagarakis, S.; Dekkers, O.M. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2016, 175, G1–G34.
- Kolomecki, K.; Stepien, H.; Bartos, M.; Kuzdak, K. Usefulness of VEGF, MMP-2, MMP-3 and TIMP-2 serum level evaluation in patients with adrenal tumours. Endocr. Regul. 2001, 35, 9–16.
- Zacharieva, S.; Atanassova, I.; Orbetzova, M.; Nachev, E.; Kalinov, K.; Kirilov, G.; Shigarminova, R.; Ivanova, R.; Dashev, G. Circulating vascular endothelial growth factor and active renin concentrations and prostaglandin E2 urinary excretion in patients with adrenal tumours. Eur. J. Endocrinol. 2004, 150, 345–349.
- Bernini, G.P.; Moretti, A.; Bonadio, A.G.; Menicagli, M.; Viacava, P.; Naccarato, A.G.; Iacconi, P.; Miccoli, P.; Salvetti, A. Angiogenesis in Human Normal and Pathologic Adrenal Cortex. J. Clin. Endocrinol. Metab. 2002, 87, 4961–4965.
- Kroiss, M.; Reuss, M.; Kühner, D.; Johanssen, S.; Beyer, M.; Zink, M.; Hartmann, M.F.; Dhir, V.; Wudy, S.A.; Arlt, W.; et al. Sunitinib Inhibits Cell Proliferation and Alters Steroidogenesis by Down-Regulation of HSD3B2 in Adrenocortical Carcinoma Cells. Front. Endocrinol. 2011, 2, 27.
- de Fraipont, F.; El Atifi, M.; Gicquel, C.; Bertagna, X.; Chambaz, E.M.; Feige, J.J. Expression of the Angiogenesis Markers Vascular Endothelial Growth Factor-A, Thrombospondin-1, and Platelet-Derived Endothelial Cell Growth Factor in Human Sporadic Adrenocortical Tumors: Correlation with Genotypic Alter. J. Clin. Endocrinol. Metab. 2000, 85, 4734–4741.
- Xu, Y.Z.; Zhu, Y.; Shen, Z.J.; Sheng, J.Y.; He, H.C.; Ma, G.; Qi, Y.C.; Zhao, J.P.; Wu, Y.X.; Rui, W.B.; et al. Significance of heparanase-1 and vascular endothelial growth factor in adrenocortical carcinoma angiogenesis: Potential for therapy. Endocrine 2011, 40, 445–451.
- Diaz-Cano, S.J.; De Miguel, M.; Blanes, A.; Galera, H.; Wolfe, H.J. Contribution of the microvessel network to the clonal and kinetic profiles of adrenal cortical proliferative lesions. Hum. Pathol. 2001, 32, 1232–1239.
- Sasano, H.; Ohashi, Y.; Suzuki, T.; Nagura, H. Vascularity in human adrenal cortex. Mod. Pathol. 1998, 11, 329–333.
- Zhu, Y.; Xu, Y.; Chen, D.; Zhang, C.; Rui, W.; Zhao, J.; Zhu, Q.; Wu, Y.; Shen, Z.; Wang, W.; et al. Expression of STAT3 and IGF2 in adrenocortical carcinoma and its relationship with angiogenesis. Clin. Transl. Oncol. 2014, 16, 644–649.
- Pereira, S.S.; Costa, M.M.; Guerreiro, S.G.; Monteiro, M.P.; Pignatelli, D. Angiogenesis and Lymphangiogenesis in the Adrenocortical Tumors. Pathol. Oncol. Res. 2018, 24, 689–693.
- Logie, J.J.; Ali, S.; Marshall, K.M.; Heck, M.M.S.; Walker, B.R.; Hadoke, P.W.F. Glucocorticoid-mediated inhibition of angiogenic changes in human endothelial cells is not caused by reductions in cell proliferation or migration. PLoS ONE 2010, 5, e14476.
