Humic substances are a very important part of our soil. The topic is description of the structure of humic substances using NMR. The NMR part is divided into two parts, liquid and solid state NMR. The assignment of NMR spectra are discussed and the structural elements that can be deduced from the spectral information. Principal Component Analysis is used as a tool to categorize the information. Structural models are discussed.
- humic acid
- fulvic aicd
- humins
- 13C-NMR
- 1H-NMR
- models
- PCA
NMR of complex, heterogeneous structures exemplied by humic substances
Poul Erik Hansen, Department of Science and Environment, Roskilde University, Roskilde, Denmark
E-mail: poulerik@ruc.dk
Humic substances have attracted very much attention due to it being the most abundant organic material on earth and because of its vital role for soil properties, as absorber of pesticides and polycyclic aromatics [1]and in that capacity as protector of underground drinking reservoirs. Humic substances can be classified in three main categories, fulvic acid (water soluble), humic acid (soluble in base) and humins (insoluble).
Considering the complexity of the problem, no simple nor uniform approach can be outlined. Many different routes have been explored. NMR of humic substances have been recorded either using solid state NMR or liquid state NMR solvent either heavy water [2] or DMSO.[3] Does it at all make sense to investigate such a heterogeneous material? Yes, fulvic acids can clearly be distinguished from humic acids and humin. Furthermore, humic acids from histosols seems to be very similar all over the world. Furthermore, Garcia et al.[4] concludes that HA from histosols (C4 plants) and composted material (C3 pants) are similar. Humic acids from other sources such as rice paddies, [5] ponds and underground water reservoirs [6] or coal sources e.g. Sigma Aldrich humic acid 2 can also be distinguished and described.
No attempt is made in the present text to give a historic overview. The aim is to give a state-of –the art description. An excellent review on Solid State NMR of natural organic matter including humic substances. [7] An overview of mainly Russian papers is given by Polyakov and Abakumov. [8]
NMR data have to be complemented with other data such as elemental analysis, Infra Red spectroscopy, Mass spectrometry, Size Exclusion chromatography and also titrations to reveal the type and number of titratable protons from mainly phenols and carboxylic acids. For an example of the latter see Ref. 2. An indispensable tool is multivariate analysis (see later).
Liquid state NMR
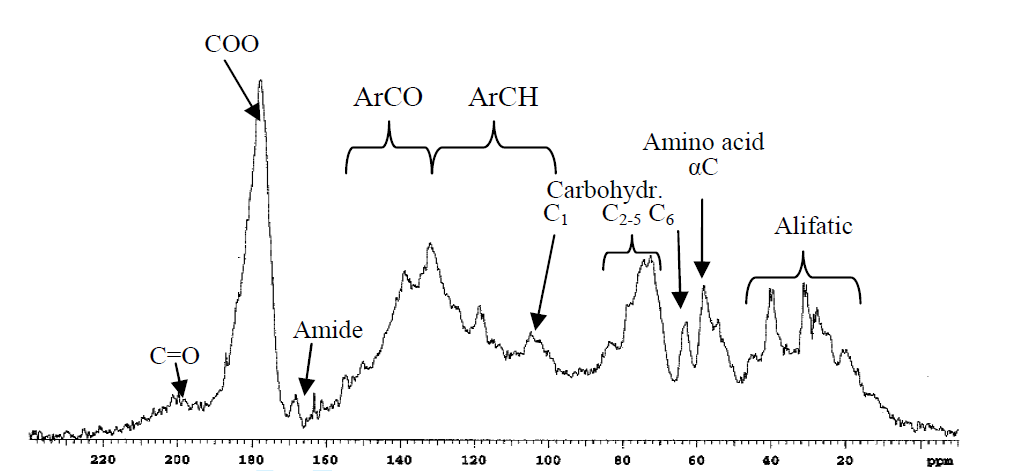
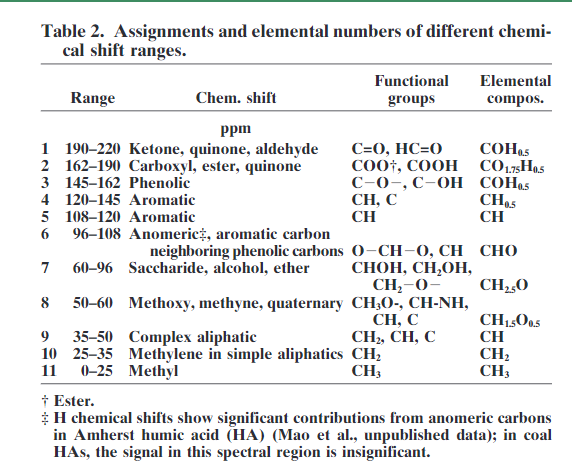
As fulvic acids are water soluble, spectra can be measured in D2O, for humic acids alkaline D2O is needed or DMSO-d6. 3 For humins solid state NMR is necessary, see later. The use of liquid state NMR has the advantage that it gives sharper lines than solid state NMR, but also that 1H NMR can be studied. Both 1D and 2D NMR techniques such as HETCOR, HMQC or HSQC [9], [10], [11], [12] been used. A correlation between 1H and 13C chemical shifts is obtained, but these parameters shows that the 1H and 13C chemical shifts are largely proportional as has been found in general [13], but in some cases such spectra have proved useful, e.g. in identifying substructures of the aromatic parts of HA and FA [9]. One of the drawback of HMBC spectra is the many resonances. The extra information of such spectra is to some extent counteracted by the increased overlap (see e.g. Ref. 3). Also combinations of pulse schemes such as DEPT-HSQC, PSGSE-NOESY, PFGSE-TOCSY and TOE-DE with diffusion editing have been used.[14] The range of 13C resonances is shown in Table “2”. A typical 1D solution 13C spectrum is shown in Fig. 1.
13C NMR
Figure 1. Liquid state spectrum of humic acid in D2O. Taken from Ref. 2 with permission from John Willey and Sons.
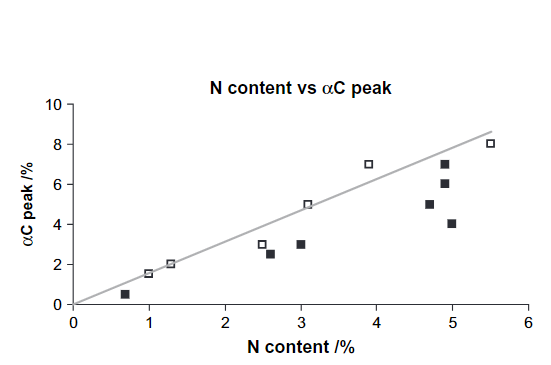
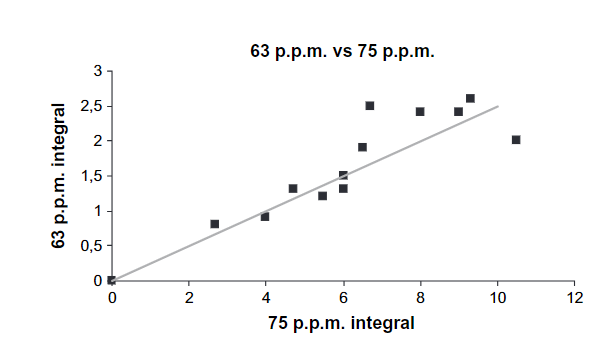
Fig. 1 shows the ranges based on tabulated data but also more specifically a peak for α-carbon of amino acids based on a correlation between %N and the percentage of C-α peak. (see Fig. 2). Furthermore, the intensities of resonances at 63 ppm are proportional to those at 75 ppm (Fig. 3) showing that this most likely is C6 (63 ppm) and C2-5 (75 ppm) of a sugar. However, looking at the signal marked C1, this seems very broad and too intense. This probably means that aromatic resonances are also present in this area. A very low chemical shift is obtained for a carbon ortho to two OR substituent and one para OR substituent (chemical shift ~94 ppm). Obviously, two OR substituents will give rise to a signal a little above 100 ppm.
Figure 2. Plot of the percentage of the C-α peak of amino acids vs. the total nitrogen content. Taken from Ref. 2 with permission from John Willey and Sons.
Figure 3. Integral of peak at 63 ppm vs. that at 75 ppm. Taken from Ref. 2 with permission from John Willey and Sons.
Taken from Ref. [15] with permission from Soil Science Society of America
In complex structures like humic substances, it is very important to have as many selective tools. One is the quantification of isolated methyl groups as proposed by Lambert and Buddrus. They ended up using a standard spin-echo technique. [16]
1H Liquid state NMR spectra.
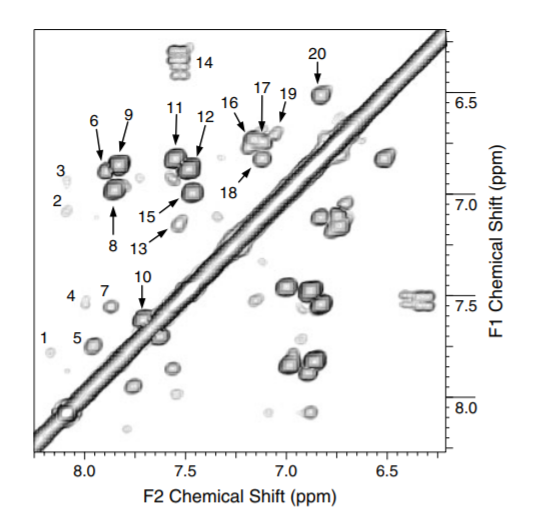
1H NMR spectra recorded in DMSO-d6 show much sharper lines than those in D2O. A fine example is shown in Fig. 4. Based on the COSY spectrum and use of databases a number of lignin like structures could be suggested. [17]
Fig. 4. COSY spectrum of fulvic acid dissolved in DMSO-d6 from Ref. 17 with permission from John Willey and Sons.
NOESY spectra have also shown resonances close to each other yielding information about the overall structure.
Solid state NMR
13C NMR
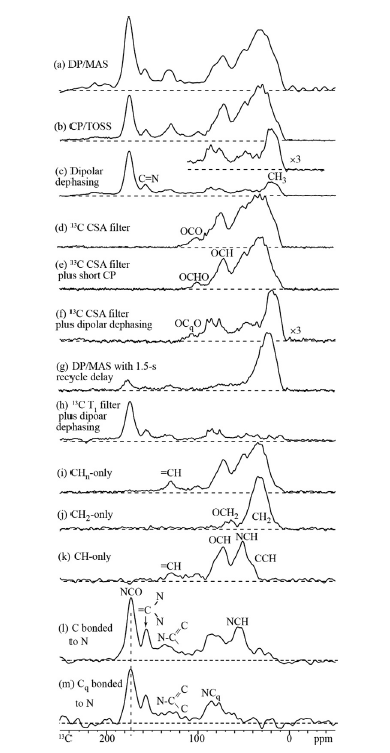
Solid state NMR is mainly using 13C NMR in a cross-polarization magic angle spinning way. However, 14,15N NMR can also be used (see later). Solid state experiments can be used to correlate 13C and 1H resonances as shown in Fig. 5 using different contact times emphasizing different types. CH carbons are seen clearly at short contact times, whereas carbons away from hydrogen require longer contact times. More elaborate schemes are also shown in Fig. 5. This enables a useful characterization of types of carbons, and a specific assignment. 13C chemical shift tables or NMR data bases are also invaluable in this context.
nitrogen.
Fig. 5. Schemes for identification types of carbons. Taken from Ref. [18] with permission from Elsevier.
Carbons bound to nitrogen can be detected using the Spider NMR Method. [19],[20] This is demonstrated in Fig. 5 l and m pictures. It is seen, that one can separate those carbons bonded to H and to N from quaternary carbons bonded to N only. Some information can be obtained by using a probe e.g. fully deuterium and 13C=O labelled benzophenone. [21]
1H NMR
High-resolution-magic angle spinning spectra may be used to observed 1H NMR spectra of humic substances attached to minerals as demonstrated by Mitchell et al. [22]
Principal component analysis
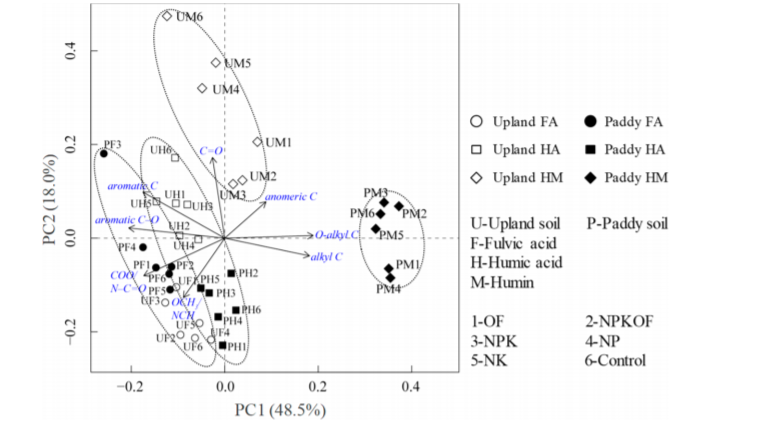
Principle component analysis is invaluable in the characterization of the inherent properties of humic substances. An example of comparison of humic acids and fulvic acids of different origin is given in Ref. 6. Another example is seen in Fig. 6 showing how humins fall in a categories of their own. [23]
Fig. 6 . Biplot generated by principal component analysis for the functional groups of fulvic acids, humic acids, and humin fractions. Taken from Ref. 23 with permission from the American Chemical Society.
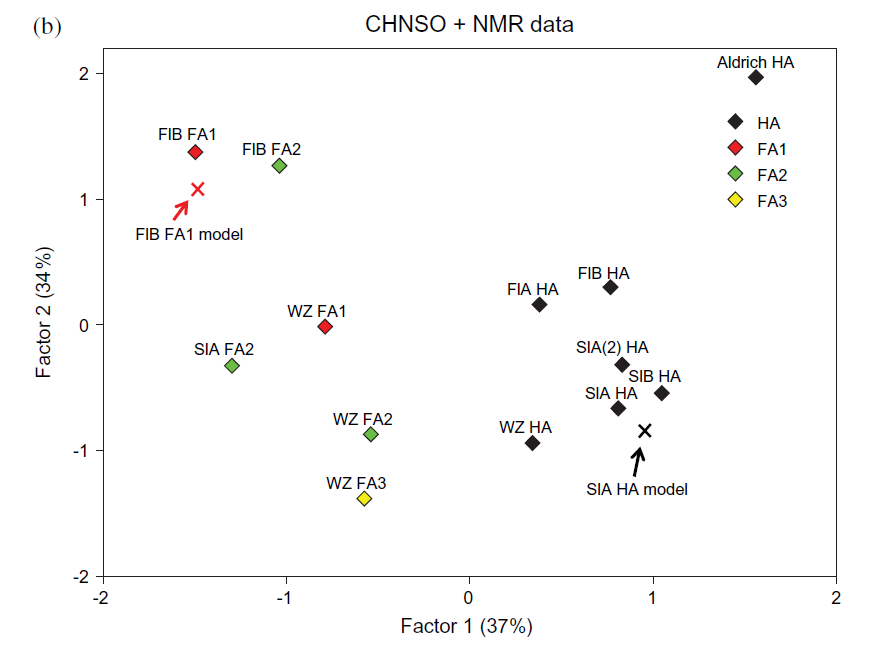
It is also interesting to see how the simulated spectra (see Modelling) fits with the experimental data as seen in Fig. 7. In this figure both a series of humic acids (HA) and fulvic acids (FA) are included. The model for this humic acid is shown in Fig. 10 (see later). Calculated factor scores for the humic and fulvic acid structural model data are marked with crosses.
Fig. 7. Scatter plots of factor scores for thirteen HS fractions based on CHNSO and 13C-NMR data. Taken from Ref. 2 with permission from John Willey and Sons.
Models
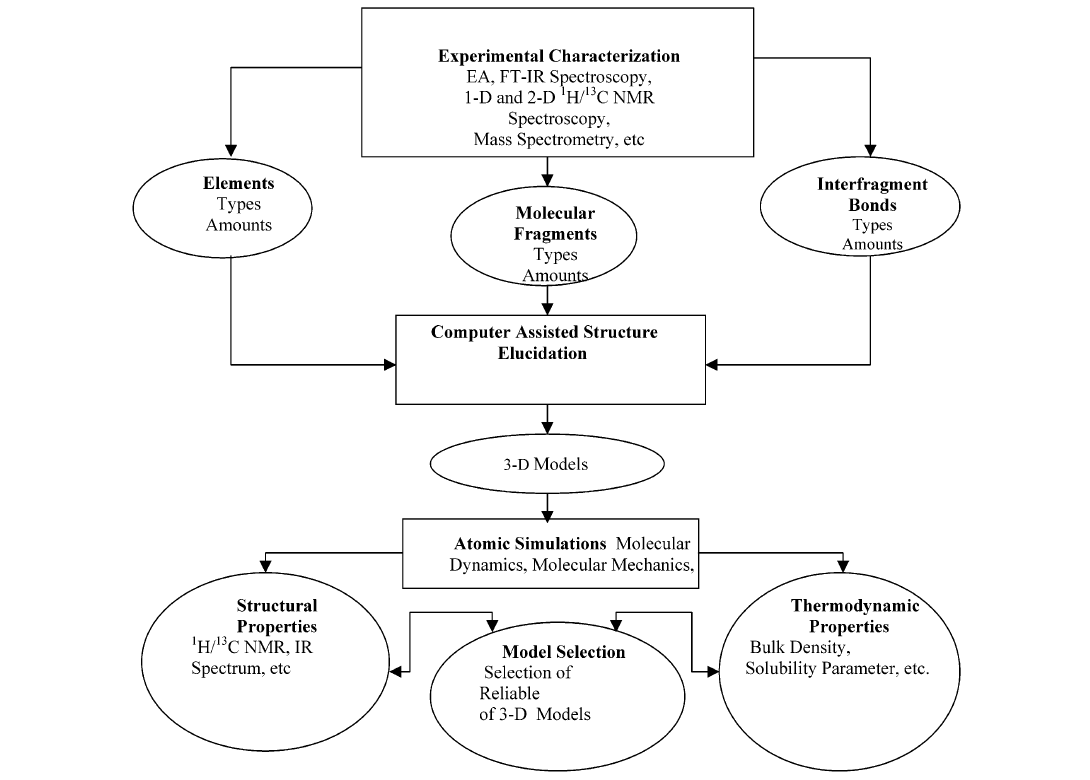
Models have been constructed for many years, but not necessarily based on NMR data. For an overview see Ref. [24]. Diallo et al. [11] constructed an elaborate scheme for construction of structures based on primarily NMR results, see Fig. 8.
Fig. 8. The hierarchical approach for modeling the 3-D structures of humic substances. Taken from Ref. 11 with permission from the American Chemical Society.
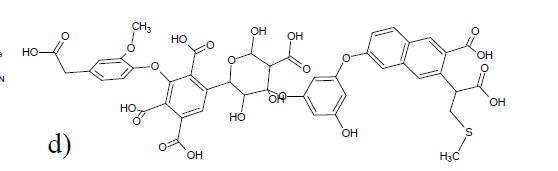
Fig. 9. One of five substructures proposed by Diallo et al. 11. Reproduced with permission from the American Chemical Society.
Diallo et al. 11 assumed based on electrospray ionization mass spectra an average molecular weight of 1016 and a formula of C45H43O24N1S1.
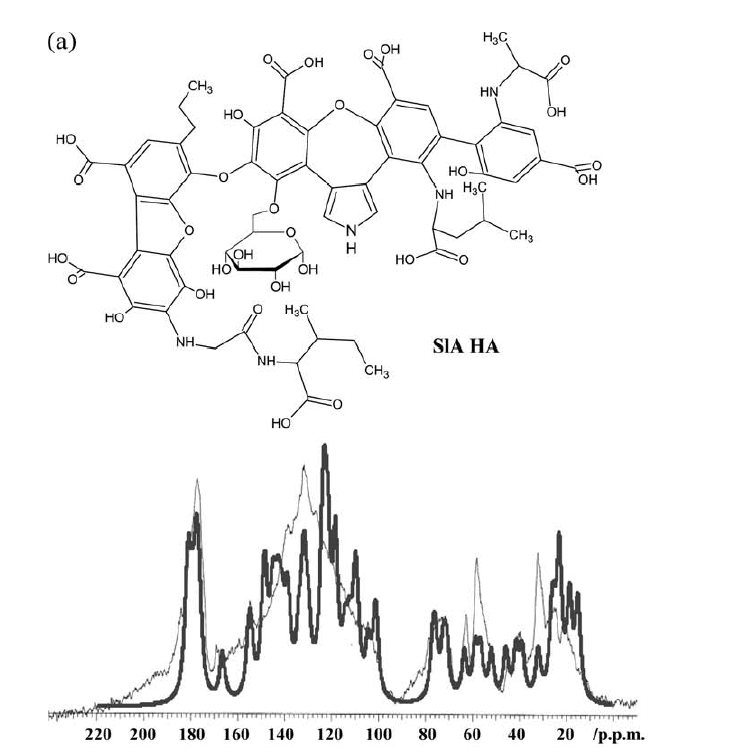
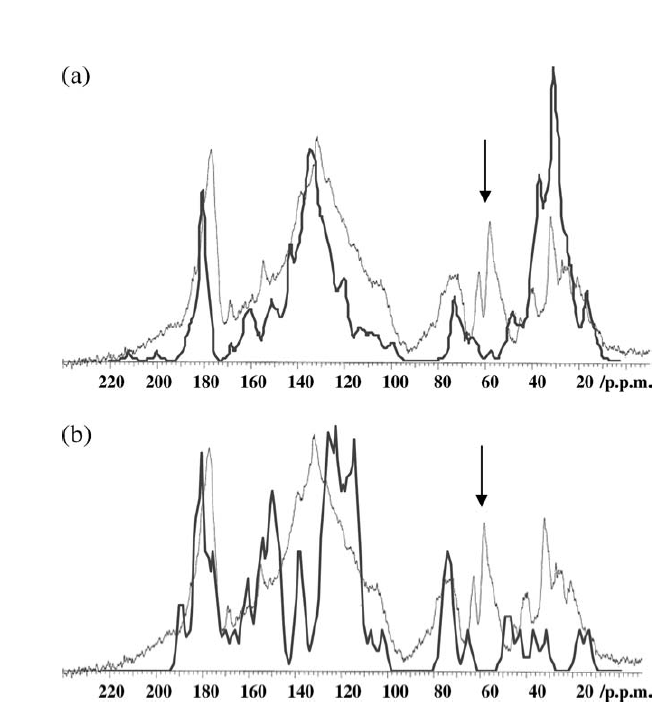
A different approach to modeling was taken by Albers et al. [2, 24]. Structures were constructed based on structural elements based on 13C chemical shifts, elemental analysis data and titration data. In that case the formula ended up as C65H67O30N5. [24] (see Fig. 10). Liquid state 13C NMR spectra were then simulated based on chemical shifts from the NMR database, NMR predict[25] taking into account slight variations due to titration of carboxyl acid groups (see Fig. 10). For prediction of a different humic acid see Ref. 11. This kind of simulation could also be used to test already suggested structures (Fig. 11). This enables to classify some of the proposed structures as not fully correct as seen in Fig. 11. and improvements could be suggested as discussed in Ref. 24.
Fig. 10. Above structure suggested by Albers et al. and below the measured 13C NMR spectrum (thin line) overlayed by the calculated 13C NMR spectrum. Taken from Ref. 2 with permission from John Willey and Sons.
The model shown in Fig. 10 is an average structure and should not be taken as the final answer but shows structural elements that can be combined in different fashions.
Fig. 11. Simulated 13C NMR spectrum (heavy line) on top of humic acid spectrum a. Simulation based on structure of Schulten and Schnitzer Ref. [26]. b. Stevenson Ref. [27]. Taken from Ref. 2 with permission from John Willey and Sons.
[1] S. Chianese , A. Fenti, P.Iovino, D. Musmarra and S. Salvestrini, Sorption of Organic Pollutants by Humic Acids: A Review. Molecules, 2020, 25, 918.
[2] C.N. Albers, G.T. Banta, O.S. Jacobsen and P.E. Hansen. Characterisation and structural modelling of Danish field humic substances displaying significant differences compared to previously proposed structures. Eur. J. Soil Sci. 2008, 59, 693-705.
[3] A.J. Simpson, B. Lefebre, A. Moser, A. Williams, N. Larin, M. Hvasna, W.L. Kigery, B. Kelleher. Identifying residues in natural organic matter through spectral prediction and pattern matching of 2D NMR datasets. Magn. Reson.Chem. 2004; 42, 14-22.
[4] A.C. Garcia, L.G.A de Souza, M.G. Pereira, R.N .Castro, J.M. Garcia-Mina, E. Zonta, F. Junior, G. Lisboa and R.L.L. Berbara, Structure-Property-Function Relationship in Humic Substances to Explain the Biological Activity in Plants, Sci-Rep. 2016, 6, 20798.
[5] P. Liu, W. Zhou, H. Cui, J. Tan and S. Cao, Structural characteristics of humic substances in buried ancient paddy soils as revealed by 13C NMR spectroscopy. Environ. Geochem. Health 2019, 41, 2459–2472
[6] M. Thomsen, P. Lassen, S. Dobel, P.E. Hansen, L.Carlsen and B.B. Mogensen. Characterisation of humic substances of different origin: a multivariate approach for quantifying the latent properties of dissolved organic matter. Chemosphere, 2002, 49, 1327-1337.
[7] J. Mao, X. Cao, D. C. Olk, W. Chu , K. Schmidt-Rohr Advanced solid-state NMR spectroscopy of natural organic matter. Prog. Magn.Reson. 2017, 100, 17-51.
[8] V. Polyakov and E. V. Abakumov, Humic Acids Isolated from Selected Soils from the Russian Arctic and Antarctic: Characterization by Two-Dimensional 1H-13C HETCOR and 13C CP/Mas NMR Spectroscopy. Geosciences, 2020, 10, 15.
[9] S. Haiber, H. Herzog, J. Buddrus, P. Burba, J. Lambert. Quantification of substructures of refractory organic substances by means of nuclear magnetic resonance. In: Frimmel FH, Abbt-Braun G, Heumann KG, Hock B, Lüdemann H-D, Spiteller M, Eds. Weinheim: Wiley-VCH, 2002; pp. 115-28.
[10] A.J. Simpson, J. Burdon, C.L. Graham, M.H.B. Hayes, N. Spencer, W.L. Kingery. Interpretation of heteronuclear and multidimensional NMR spectroscopy of humic substances, Eur. J. Soil. Sci. 2001, 52, 495-509.
[11] M.S. Diallo, A. Simpson, P. Gassman, J.H. Johnson, W.A. Goddard, P.G. Hatcher. 3-D structural modelling of humic acids through experimental characterization, computer assisted structure elucidation and atomistic simulations. 1. Chelsea soil humic acid. Environ. Sci. Technol. 2003, 37, 1783-93.
[12] J.-D. Mao and K. Schmidt-Rohr. Absence of mobile carbohydrate domains in dry humic substances proven by NMR, and implications for organic-contaminant sorption models. Environ. Sci. Technol. 2006, 40, 1751-56.
[13] R.S. Macomber. Proton-Carbon Chemical Shift Correlations. J. Chem. Edu. 1991, 68, 284-85.
[14] A.J. Simpson, G. Song, E. Smith, B. Lam, E.H. Novotny and M.H.B. Hayes, Unraveling the Structural Components of soil Humin by Use of solution-State Nuclear Magnetic Resonance spectroscopy, Envir.Sci.Technol. 2007, 41, 876-883.
[15] J.-D. Mao, W.G. Hu, K. Schmidt-Rohr, G. Davies, E.A. Ghabbour, B.S. Xing, Quantitative characterization of humic substances by solid-state carbon-13 nuclear magnetic resonance, Soil Sci. America J. 2000, 64, 873-884.
[16] J. Lambert and J. Buddrus, Quantification of Isolated Methyl groups in Aquatic Humic Substances by Means of 1H and 13C NMR spectroscopy. Magn.Reson.Chem. 1996, 34, 276-282.
[17] A. J. Simpson, B. Lefebvre, A. Moser, A. Williams, N. Larin, M. Kvasha, W. L. Kingery and B. Kelleher. Identifying residues in natural organic matter through spectral prediction and pattern matching of 2D NMR datasets. Magn.Reson.Chem. 2004, 42,14-22.
[18] J.-D. Mao, R.M. Cory, D.M. McKnight, K. Schmidt-Rohr, Characterization of a nitrogen-rich fulvic acid and its precursor algae by solid-state NMR, Org. Geochem. 2007, 38, 1277–1292.
[19] K. Schmidt-Rohr, J.D. Mao, D.C. Olk, Nitrogen-bonded aromatics in soil organic matter and their implications for a yield decline in intensive rice cropping, Proc. Nat. Acad. Sci. USA 2004, 101, 6351–6354.
[20] J.-D. Mao, R.M. Cory, D.M. McKnight, K. Schmidt-Rohr, Characterization of a nitrogen-rich fulvic acid and its precursor algae by solid-state NMR, Org. Geochem. 2007, 38, 1277–1292.
[21] X. Cao, C. Lattao, J. J. Pignatello, J. Mao and K. Schmidt-Rohr. Sorption Selectivity in Natural Organic Matter Probed with Fully Deuterium-Exchanged and Carbonyl-13C‑Labeled Benzophenone and 1H−13C NMR Spectroscopy. Environ. Sci. Technol. 2014, 48, 8645−8652.
[22] P.J. Mitchell , A. J. Simpson, R. Soong and M. J. Simpson. Nuclear Magnetic Resonance Analysis of Changes in Dissolved Organic Matter Composition with Successive Layering on Clay Mineral Surfaces. Soil Systems, 2018, 2, 8.
[23] J. Xu, B. Zhao, Z. Li, W.Chu, J. Mao, D. C. Olk, J. Zhang, X. Xin, and W. Wei. Demonstration of Chemical Distinction among Soil Humic Fractions Using Quantitative Solid-State 13C NMR J. Agric. Food Chem. 2019, 67, 8107−8118.
[24] C. N. Albers, P.E. Hansen, 13C-NMR chemical shift databases as a quick tool to evaluate structural models of humic substances. Open.Magn.Reson.J. 2010, 3 , 96-105.
[25] NMR predict. Modgraph Consultants Ltd.
[26] H.-R Schulten and M. Schnitzer, A state of the art structural concept for humic substances. Naturwissenschaften, 1993, 80, 9-30.
[27] F.J. Stevenson, Humus Chemistry: Genesis, Composition, Reactions. John Willey and Sons, New York. 1994.