Atherosclerosis refers to the pathophysiological conditions where the medium and large arteries are significantly affected due to spatial deposition of various materials such as lipoproteins (particularly cholesterol), immune cells (circulating monocytes), proinflammatory factors (i.e., macrophages and T-cells), degraded extracellular matrix components and necrotic products. Atherosclerosis is considered to be the primary cause of chronic CVDs, including coronary heart disease, cerebrovascular disease, and peripheral arterial disease [38,39,40]. Atherosclerosis is characterized by a series of physiological events, including endothelial dysfunction, inflammatory responses, cell proliferation, lipoprotein deposition, vascular remodeling, and finally, plaque formation.
Atherosclerosis refers to the pathophysiological conditions where the medium and large arteries are significantly affected due to spatial deposition of various materials such as lipoproteins (particularly cholesterol), immune cells (circulating monocytes), proinflammatory factors (i.e., macrophages and T-cells), degraded extracellular matrix components and necrotic products. Atherosclerosis is considered to be the primary cause of chronic CVDs, including coronary heart disease, cerebrovascular disease, and peripheral arterial disease. Atherosclerosis is characterized by a series of physiological events, including endothelial dysfunction, inflammatory responses, cell proliferation, lipoprotein deposition, vascular remodeling, and finally, plaque formation.
- diagnosis
- bioimaging
- therapeutics
- nanotheranostics
- CVDs
1. Nanotheranostics for Atherosclerosis
1.1. Atherosclerosis: An Overview
Atherosclerosis refers to the pathophysiological conditions where the medium and large arteries are significantly affected due to spatial deposition of various materials such as lipoproteins (particularly cholesterol), immune cells (circulating monocytes), proinflammatory factors (i.e., macrophages and T-cells), degraded extracellular matrix components and necrotic products. Atherosclerosis is considered to be the primary cause of chronic CVDs, including coronary heart disease, cerebrovascular disease, and peripheral arterial disease [38,39,40]. Atherosclerosis is characterized by a series of physiological events, including endothelial dysfunction, inflammatory responses, cell proliferation, lipoprotein deposition, vascular remodeling, and finally, plaque formation [41]. The pathophysiological conditions associated with atherosclerosis are highly influenced by the macrophages as their migration, activation, infiltration and proliferation characteristically promote the formation of inflammated atherosclerotic plaques. The atherosclerotic plaques are composed of lipids (mainly cholesterol followed by phospholipids), inflammatory cells, smooth muscle cells, connective tissue (including collagen and elastic fibers), thrombi and calcium deposits [42]. The inflammation is due to the secretion of several proteases and tissue factors under macrophages’ influence. Hence, macrophage-targeted therapeutics are considered to be promising in minimizing the inflammatory responses and the degradation of atherosclerotic plaques [39]. The design and development of theranostic nanomaterials for the management of atherosclerosis depend upon several physiological markers. One of the exciting biomarkers to target atherosclerotic plaques is fibrin, which characteristically facilitates sensitive detection during plaque formation. In addition to fibrin, α
Atherosclerosis refers to the pathophysiological conditions where the medium and large arteries are significantly affected due to spatial deposition of various materials such as lipoproteins (particularly cholesterol), immune cells (circulating monocytes), proinflammatory factors (i.e., macrophages and T-cells), degraded extracellular matrix components and necrotic products. Atherosclerosis is considered to be the primary cause of chronic CVDs, including coronary heart disease, cerebrovascular disease, and peripheral arterial disease [1][2][3]. Atherosclerosis is characterized by a series of physiological events, including endothelial dysfunction, inflammatory responses, cell proliferation, lipoprotein deposition, vascular remodeling, and finally, plaque formation [4]. The pathophysiological conditions associated with atherosclerosis are highly influenced by the macrophages as their migration, activation, infiltration and proliferation characteristically promote the formation of inflammated atherosclerotic plaques. The atherosclerotic plaques are composed of lipids (mainly cholesterol followed by phospholipids), inflammatory cells, smooth muscle cells, connective tissue (including collagen and elastic fibers), thrombi and calcium deposits [5]. The inflammation is due to the secretion of several proteases and tissue factors under macrophages’ influence. Hence, macrophage-targeted therapeutics are considered to be promising in minimizing the inflammatory responses and the degradation of atherosclerotic plaques [2]. The design and development of theranostic nanomaterials for the management of atherosclerosis depend upon several physiological markers. One of the exciting biomarkers to target atherosclerotic plaques is fibrin, which characteristically facilitates sensitive detection during plaque formation. In addition to fibrin, α
v
β
3 integrin is also considered a unique biomarker as it is associated with the process of angiogenesis and transiently expressed when the endothelial layer remains physiologically intact. Hence, the therapeutic measures centered around targeting these important physiological markers when spatially designed nanomaterials are being considered as theranostic materials [43]. Looking into the conventional therapeutics for atherosclerosis, it was observed that drug candidates targeting atherosclerosis promoting factors such as hypertension and dyslipidemias are regularly being considered. In addition, the importance of inflammatory responses during atherosclerotic plaque formation, anti-inflammatory drugs are also being administered to treat atherosclerosis [38]. Additionally, the conventional therapeutics also include FDA-approved statins i.e., β-Hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase inhibitors), fibrates, nicotinic acid and ezetimibe [44].
integrin is also considered a unique biomarker as it is associated with the process of angiogenesis and transiently expressed when the endothelial layer remains physiologically intact. Hence, the therapeutic measures centered around targeting these important physiological markers when spatially designed nanomaterials are being considered as theranostic materials [6]. Looking into the conventional therapeutics for atherosclerosis, it was observed that drug candidates targeting atherosclerosis promoting factors such as hypertension and dyslipidemias are regularly being considered. In addition, the importance of inflammatory responses during atherosclerotic plaque formation, anti-inflammatory drugs are also being administered to treat atherosclerosis [1]. Additionally, the conventional therapeutics also include FDA-approved statins i.e., β-Hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase inhibitors), fibrates, nicotinic acid and ezetimibe [7].
1.2. Nano-Based Platforms for Diagnosis and Treatment of Atherosclerosis
The concept of nano-based platforms represents the basis for multifaceted applications such as diagnosis of pathophysiological conditions of any particular disease and critically modulating the therapeutic measures from a single platform. The nano-based theranostics are regularly used in cancer diagnosis and therapies. The promising theranostic aspects of nano-based cancer therapy platforms gained considerable interest in the last few years for targeted image-guided treatment of other chronic diseases, including CVDs. Spatially designed nano-systems are considered the highly decorated arsenal to treat atherosclerosis [36] (
The concept of nano-based platforms represents the basis for multifaceted applications such as diagnosis of pathophysiological conditions of any particular disease and critically modulating the therapeutic measures from a single platform. The nano-based theranostics are regularly used in cancer diagnosis and therapies. The promising theranostic aspects of nano-based cancer therapy platforms gained considerable interest in the last few years for targeted image-guided treatment of other chronic diseases, including CVDs. Spatially designed nano-systems are considered the highly decorated arsenal to treat atherosclerosis [8] (
). As per the recent trends, FDA-approved nanoparticles such as PLGA, hyaluronic acid, and liposomes gained considerable attention for atherosclerosis treatment. These nanomaterials tend to imitate high-density lipoproteins (HDLs), which are known for anti-atherogenic properties.
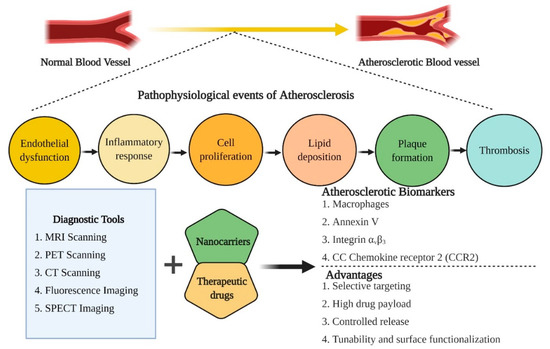
Figure 1.
An overview of the pathophysiological events associated with the induction of atherosclerosis, therapeutic biomarkers for diagnosis and treatment of atherosclerosis and the advantages associated with nano-based theranostic tools for the management of CVDs.
Similarly, polymeric nanoparticles and HDL mimicking nanoparticles have also gained the center of attraction for determining the bioimaging and therapeutic modalities in managing atherosclerosis [45]. In the quest for novel nanotherapeutics for diagnosis and treatment of atherosclerosis, a biologically active natural polymer, hyaluronic acid (HA), was exploited for nanoparticle synthesis. The amine-functionalized hyaluronic acid-nanoparticles (HA-NPs) labeled with a fluorescent or radionuclide agent effectively promote macrophages’ logistic uptake and mediate ablation of atherosclerotic plaques. The fluorescent agent presence served as a potential contrasting agent and provided a platform for bioimaging modalities [46]. In the last decade, a significant contribution has been made in the field of designing and developing novel nano-based theranostic agents for the diagnosis and therapy of atherosclerotic plaques through the common platform (
Similarly, polymeric nanoparticles and HDL mimicking nanoparticles have also gained the center of attraction for determining the bioimaging and therapeutic modalities in managing atherosclerosis [9]. In the quest for novel nanotherapeutics for diagnosis and treatment of atherosclerosis, a biologically active natural polymer, hyaluronic acid (HA), was exploited for nanoparticle synthesis. The amine-functionalized hyaluronic acid-nanoparticles (HA-NPs) labeled with a fluorescent or radionuclide agent effectively promote macrophages’ logistic uptake and mediate ablation of atherosclerotic plaques. The fluorescent agent presence served as a potential contrasting agent and provided a platform for bioimaging modalities [10]. In the last decade, a significant contribution has been made in the field of designing and developing novel nano-based theranostic agents for the diagnosis and therapy of atherosclerotic plaques through the common platform (
).
Table 1.
List of notable theranostic nanoparticles designed and developed for the diagnosis and therapy of atherosclerosis, an important factor of cardiovascular diseases (CVDs) since the 2010s.
| Sl. No. | Theranostic Agents | Bioimaging Modalities | Therapeutic Modalities | Target | References | |||
|---|---|---|---|---|---|---|---|---|
| 1. | Cross-linked dextran-coated iron oxide (CLIO) magnetofluorescent nanoparticles |
Fluorescence imaging/MRI | meso-tetra( | m | -hydroxyphenyl)chlorin (THPC) | Macrophagic ablation in atherosclerosis | [47] | [11] |
| 2. | Protease-mediated theranostic agent | Near-infrared fluorescence imaging | Cathepsin-B activatable theranostic agent (L-SR15) | Plaque-destabilizing Cathepsin-B activity by selectively eliminating macrophages |
[48] | [12] | ||
| 3. | Gold nanorods | Computed tomography (CT) imaging | Photothermally active near-infrared irradiation | Ablation of inflammatory macrophages | [49] | [13] | ||
| 4. | Silica coated plasmonic gold nanorods | Combined intravascular ultrasound (IVUS) and intravascular photoacoustic (IVPA) imaging | Continuous-wave laser | Atherosclerotic plaque management | [50] | [14] | ||
| 5. | Single-walled Carbon nanotubes (SWCNTs) | Near-infrared fluorescence imaging | Photothermally active near-infrared irradiation | Macrophagic apoptosis | [51] | [15] | ||
| 6. | 18 | F-fluorodeoxyglucose labeled liposomal glucocorticoid (L-PLP) | Positron emission tomography (PET)/ magnetic resonance imaging (MRI) | Glucocorticoid (PLP) | Management of atherosclerotic lesions | [52] | [16] | |
| 7. | Doxorubicin-loaded hyaluronic acid-polypyrrole nanoparticles | Fluorescence imaging | Doxorubicin | Targeting proliferating macrophages | [53] | [17] | ||
| 8. | High-density lipoprotein-like magnetic nanostructures | MRI | High-density lipoprotein | Reverse cholesterol transport | [54] | [18] | ||
| 9. | Hybrid lipid–latex (LiLa) nanoparticles | MRI | Rosiglitazone | Targeting proliferating macrophages in atherosclerosis | [55] | [19] | ||
| 10. | Solid-Lipid nanoparticles (SLNs) | MRI | α-tocopherol or prostacyclin (PGI2) |
Platelet aggregation | [56] | [20] |
Angiogenesis is a standard process during atherosclerosis growth and progression. Hence, anti-angiogenic factors and drug moieties are being considered therapeutic modules for atherosclerosis treatment. For more specificity, the anti-angiogenic drug, perfluorocarbon, was designed into the phospholipid bilayer-based nano-emulsions targeting the α
v
β
3 integrin and thus served as multimodal nanocomplexes for diagnosis and therapy [43]. In addition, inorganic nanoparticles, magnetic nanoparticles, polymeric nanoparticles, and micelles are also considered promising nanocarriers for target-oriented delivery of a wide range of drug moieties, antibodies and photosensitizers. One of the interesting aspects of considering nanomaterials as theranostic agents for atherosclerotic therapeutics is their tunability to utilize various bioimaging modalities to diagnose disease progression [57]. Since the nano-based platforms provide a wide range of diagnostic imaging and therapeutic applications for atherosclerosis, an array of nano-based carriers are actively exploited for vascular disease management.
integrin and thus served as multimodal nanocomplexes for diagnosis and therapy [6]. In addition, inorganic nanoparticles, magnetic nanoparticles, polymeric nanoparticles, and micelles are also considered promising nanocarriers for target-oriented delivery of a wide range of drug moieties, antibodies and photosensitizers. One of the interesting aspects of considering nanomaterials as theranostic agents for atherosclerotic therapeutics is their tunability to utilize various bioimaging modalities to diagnose disease progression [21]. Since the nano-based platforms provide a wide range of diagnostic imaging and therapeutic applications for atherosclerosis, an array of nano-based carriers are actively exploited for vascular disease management.
The characteristic physicochemical properties such as high SPR, absorption capability, optical properties, biocompatibility and ability to bind to targeting agents provide the functional attributes of gold nanoparticles (AuNPs) as bioimaging and therapeutic modules. The high SPR effect of AuNPs is being deployed for photothermal therapy (PTT), where AuNPs promote macrophage depletion by transducing the light energy into heat in atherosclerotic conditions. Inorganic nanoparticles are being actively employed for PTT utilizing spatial wavelength of near-infrared laser light, leading to intracellular hyperthermia. The use of gold nanorods and silica-gold hybrid nanoparticles was reported to work in tandem with PTT and induce a series of physiological events, including the macrophages apoptosis and reduced risk of cardiovascular mortality by decreasing the total atheroma volume, respectively [58]. AuNPs also act as promising carriers for photosensitizers during photodynamic therapy (PDT). AuNPs facilitate the binding of photosensitizers to the atherosclerotic plaques and mediates the plaques’ depletion by generating reactive oxygen species (ROS) when irradiated. The surface modification and functionalization of AuNPs further improved the therapeutic measures under consideration. The PEGylated aminolevulinic acid-coated AuNPs also exhibited a significant effect on plaque macrophage content [41].
The characteristic physicochemical properties such as high SPR, absorption capability, optical properties, biocompatibility and ability to bind to targeting agents provide the functional attributes of gold nanoparticles (AuNPs) as bioimaging and therapeutic modules. The high SPR effect of AuNPs is being deployed for photothermal therapy (PTT), where AuNPs promote macrophage depletion by transducing the light energy into heat in atherosclerotic conditions. Inorganic nanoparticles are being actively employed for PTT utilizing spatial wavelength of near-infrared laser light, leading to intracellular hyperthermia. The use of gold nanorods and silica-gold hybrid nanoparticles was reported to work in tandem with PTT and induce a series of physiological events, including the macrophages apoptosis and reduced risk of cardiovascular mortality by decreasing the total atheroma volume, respectively [22]. AuNPs also act as promising carriers for photosensitizers during photodynamic therapy (PDT). AuNPs facilitate the binding of photosensitizers to the atherosclerotic plaques and mediates the plaques’ depletion by generating reactive oxygen species (ROS) when irradiated. The surface modification and functionalization of AuNPs further improved the therapeutic measures under consideration. The PEGylated aminolevulinic acid-coated AuNPs also exhibited a significant effect on plaque macrophage content [4].
Similarly, mesoporous silica-coated conversion nanoparticles are being exploited as a platform for photosensitizers to trigger NIR-mediated PDT. Under NIR irradiation’s influence, a significant decrease in the intracellular lipids and an increased influx of cholesterol efflux were observed, suggesting an antagonistic effect on atherosclerosis [59,60]. In the early 2000s, a new therapeutic strategy evolved, known as plasmonic PTT (PPTT), which is considered for targeting the atherosclerotic plaque formation by exploiting the infrared properties of noble metal nanoparticles (specifically AuNPs) under the guidance of magnetic resonance [61]. The spatially designed multifunctional AuNPs could bring a paradigm shift in the current therapeutic intervention concept for CVDs, including atherosclerosis. The involvement of AuNPs as contrasting agents for early diagnosis through CT imaging exhibited high-density resolution of the deep tissues under consideration and could be influential in atherosclerosis bioimaging [58].
Similarly, mesoporous silica-coated conversion nanoparticles are being exploited as a platform for photosensitizers to trigger NIR-mediated PDT. Under NIR irradiation’s influence, a significant decrease in the intracellular lipids and an increased influx of cholesterol efflux were observed, suggesting an antagonistic effect on atherosclerosis [23][24]. In the early 2000s, a new therapeutic strategy evolved, known as plasmonic PTT (PPTT), which is considered for targeting the atherosclerotic plaque formation by exploiting the infrared properties of noble metal nanoparticles (specifically AuNPs) under the guidance of magnetic resonance [25]. The spatially designed multifunctional AuNPs could bring a paradigm shift in the current therapeutic intervention concept for CVDs, including atherosclerosis. The involvement of AuNPs as contrasting agents for early diagnosis through CT imaging exhibited high-density resolution of the deep tissues under consideration and could be influential in atherosclerosis bioimaging [22].
Paramagnetic nanoparticles are highly effective in the diagnosis and treatment of atherosclerosis. Anti-angiogenic factors could be encapsulated into the magnetically active nanomaterials for effective clearance of atherosclerotic plaques associated with angiogenesis. The optical properties also facilitate the bioimaging module, which further enables therapeutic measures under consideration [42]. Other inorganic nanoparticles, including iron oxide nanoparticles (IONPs), are also being exploited for the management of atherosclerosis. When iron oxide nanoparticles are used combined with conventional bioimaging modalities (i.e., MRI and PET scan), highly sensitive and highly spatial resolution were observed, suggesting their candidature for bioimaging modalities [58]. Since IONPs exhibit superparamagnetic properties (SPIONs), they could be exploited as alternative contrasting agents for conventional diagnostic imaging techniques, including MRI. The ease of synthesis, ease of biofunctionalization and chemical modification are the functional attributes of SPIONs depicting their candidature for bioimaging and therapeutics [62]. SPIONS-based multimodal imaging technique enables atherosclerosis diagnosis as it could be exploited to image the pathophysiological conditions within the macrophages and other biological moieties with high specificity [40]. Besides being used as bioimaging agents, SPIONS also provided platforms for encapsulation of drug moieties of interest, followed by guiding the drug moieties towards the target sites under the influence of magnetic fields [40]. The attributes such as surface functionalization and modification facilitate selective targeting and image-guided therapies. As discussed earlier, macrophages are considered a promising target when designing nanotheranostic platforms to manage atherosclerosis. In this context, cross-linked dextran-coated iron oxide nanoparticles (CLIO-NPs) were prepared in a combination of a chlorine-based photosensitizer, i.e., meso-tetra (
Paramagnetic nanoparticles are highly effective in the diagnosis and treatment of atherosclerosis. Anti-angiogenic factors could be encapsulated into the magnetically active nanomaterials for effective clearance of atherosclerotic plaques associated with angiogenesis. The optical properties also facilitate the bioimaging module, which further enables therapeutic measures under consideration [5]. Other inorganic nanoparticles, including iron oxide nanoparticles (IONPs), are also being exploited for the management of atherosclerosis. When iron oxide nanoparticles are used combined with conventional bioimaging modalities (i.e., MRI and PET scan), highly sensitive and highly spatial resolution were observed, suggesting their candidature for bioimaging modalities [22]. Since IONPs exhibit superparamagnetic properties (SPIONs), they could be exploited as alternative contrasting agents for conventional diagnostic imaging techniques, including MRI. The ease of synthesis, ease of biofunctionalization and chemical modification are the functional attributes of SPIONs depicting their candidature for bioimaging and therapeutics [26]. SPIONS-based multimodal imaging technique enables atherosclerosis diagnosis as it could be exploited to image the pathophysiological conditions within the macrophages and other biological moieties with high specificity [3]. Besides being used as bioimaging agents, SPIONS also provided platforms for encapsulation of drug moieties of interest, followed by guiding the drug moieties towards the target sites under the influence of magnetic fields [3]. The attributes such as surface functionalization and modification facilitate selective targeting and image-guided therapies. As discussed earlier, macrophages are considered a promising target when designing nanotheranostic platforms to manage atherosclerosis. In this context, cross-linked dextran-coated iron oxide nanoparticles (CLIO-NPs) were prepared in a combination of a chlorine-based photosensitizer, i.e., meso-tetra (
m-hydroxyphenyl) chlorin (THPC) for macrophage targeted atherosclerosis therapeutics under the influence of near-infrared light. Based upon the promising results, it could be suggested that nanocarriers-based PDT may provide an added advantage to deep tissue imaging and therapy [47,63].
-hydroxyphenyl) chlorin (THPC) for macrophage targeted atherosclerosis therapeutics under the influence of near-infrared light. Based upon the promising results, it could be suggested that nanocarriers-based PDT may provide an added advantage to deep tissue imaging and therapy [11][27].
Based upon the characteristic physicochemical properties such as biocompatibility, stability, high drug payload, sustained-release efficacy, localized drug targeting, and relatively low toxicity, the lipid-based nanoparticles, including liposomes, are being actively exploited for diagnosis and therapies of atherosclerosis. Since the liposomal carrier could incorporate both hydrophilic and hydrophobic drug molecules, encapsulation of drug moieties and antibiotics of different specificity are encapsulated into the liposomal carrier for diagnosis and/or therapy for atherosclerosis [57,64]. When combined with an anti-proliferative agent, paclitaxel, earlier, cholesterol-rich nano-emulsions exhibited a promising reduction in the size of the atherosclerotic lesions [65]. The exploration of novel hybrid nanomaterials for diagnosis and therapeutics gained recent recognition as the combination of spatial physicochemical attributes provided new dimensions to the current understanding of therapeutic measures. In this context, synthetic polymer-lipid hybrid nanoparticles were designed for the treatment of atherosclerosis. The spatially designed hybrid nanomaterials specifically target multiple target sites due to the combination of dual-responsive nanomaterials. Besides, the hybrid nanomaterials also allowed encapsulation of high SPR exhibiting inorganic nanoparticles (AuNPs, SPIONs, and QDs) as a potential contrasting agent that facilitates the bioimaging modalities of the highly designed hybrid nanomaterials. Thus, hybrid nanomaterials could provide a new basis for atherosclerosis prognosis and therapeutic management [66]. Earlier, spatially designed solid-lipid nanoparticles (SLN) conjugated with a contrasting agent (iron oxide nanoparticles) and prostacyclin exhibited a reduction in platelet aggregation and thus could be instrumental in atherosclerosis theranostics [56].
Based upon the characteristic physicochemical properties such as biocompatibility, stability, high drug payload, sustained-release efficacy, localized drug targeting, and relatively low toxicity, the lipid-based nanoparticles, including liposomes, are being actively exploited for diagnosis and therapies of atherosclerosis. Since the liposomal carrier could incorporate both hydrophilic and hydrophobic drug molecules, encapsulation of drug moieties and antibiotics of different specificity are encapsulated into the liposomal carrier for diagnosis and/or therapy for atherosclerosis [21][28]. When combined with an anti-proliferative agent, paclitaxel, earlier, cholesterol-rich nano-emulsions exhibited a promising reduction in the size of the atherosclerotic lesions [29]. The exploration of novel hybrid nanomaterials for diagnosis and therapeutics gained recent recognition as the combination of spatial physicochemical attributes provided new dimensions to the current understanding of therapeutic measures. In this context, synthetic polymer-lipid hybrid nanoparticles were designed for the treatment of atherosclerosis. The spatially designed hybrid nanomaterials specifically target multiple target sites due to the combination of dual-responsive nanomaterials. Besides, the hybrid nanomaterials also allowed encapsulation of high SPR exhibiting inorganic nanoparticles (AuNPs, SPIONs, and QDs) as a potential contrasting agent that facilitates the bioimaging modalities of the highly designed hybrid nanomaterials. Thus, hybrid nanomaterials could provide a new basis for atherosclerosis prognosis and therapeutic management [30]. Earlier, spatially designed solid-lipid nanoparticles (SLN) conjugated with a contrasting agent (iron oxide nanoparticles) and prostacyclin exhibited a reduction in platelet aggregation and thus could be instrumental in atherosclerosis theranostics [20].
Understanding the pathophysiology of atherosclerosis, it was observed that CC chemokine receptor 2 (CCR2) tends to be highly expressed on inflammatory cells and in the atherosclerotic plaques. Hence, targeting the transcriptomic profiling of CCR2 could pave the way for effective therapeutics in atherosclerosis management. In this context, self-assembled specific peptide-based nanoparticles were spatially designed to target CCR2. Since CCR2 is associated with atherosclerosis progression, the high affinity towards CCR2 could provide the platform for both diagnosis and therapeutics of atherosclerosis. The spatially designed peptide-based nanoparticles inhibited the expression of CCR2 by mediating a cascade of events such as ameliorating the monocyte migration and modulating the inflammatory response of IL-1β [67]. It is evident from pathophysiological studies, low-density lipoproteins (LDLs) naturally accumulate in the atherosclerotic plaques and associate with the proteoglycans and remnant ROS. As a result, an increased accumulation of macrophages occurs at the plaque site mediated through a number of receptors such as Toll-like receptors (TLR4), lectin-like receptors (LOX-R) and scavenger receptor A (SRA). As the LDLs primarily involve in atherosclerotic progression, squalene (precursor for cholesterol) was employed as a biomimetic carrier for specific interaction with the LDLs. When the biomimetic carrier is conjugated with specific fluorescent molecules, it could be utilized for bioimaging and image-guided therapy for atherosclerosis [68].
Understanding the pathophysiology of atherosclerosis, it was observed that CC chemokine receptor 2 (CCR2) tends to be highly expressed on inflammatory cells and in the atherosclerotic plaques. Hence, targeting the transcriptomic profiling of CCR2 could pave the way for effective therapeutics in atherosclerosis management. In this context, self-assembled specific peptide-based nanoparticles were spatially designed to target CCR2. Since CCR2 is associated with atherosclerosis progression, the high affinity towards CCR2 could provide the platform for both diagnosis and therapeutics of atherosclerosis. The spatially designed peptide-based nanoparticles inhibited the expression of CCR2 by mediating a cascade of events such as ameliorating the monocyte migration and modulating the inflammatory response of IL-1β [31]. It is evident from pathophysiological studies, low-density lipoproteins (LDLs) naturally accumulate in the atherosclerotic plaques and associate with the proteoglycans and remnant ROS. As a result, an increased accumulation of macrophages occurs at the plaque site mediated through a number of receptors such as Toll-like receptors (TLR4), lectin-like receptors (LOX-R) and scavenger receptor A (SRA). As the LDLs primarily involve in atherosclerotic progression, squalene (precursor for cholesterol) was employed as a biomimetic carrier for specific interaction with the LDLs. When the biomimetic carrier is conjugated with specific fluorescent molecules, it could be utilized for bioimaging and image-guided therapy for atherosclerosis [32].
In recent times, considerable interest is being laid upon using naturally derived molecules, mostly from plant sources, for therapeutic applications. Plant-based natural products are also observed for the synthesis of biogenic nanoparticles. Besides, plant-based compounds are also spatially incorporated into specific nanocarriers as effective therapeutics. In this context, plant-based compounds loaded into nanocarriers also gained considerable attention for the treatment of vascular diseases, including CVDs, as it is evident that inorganic nanoparticles are being actively used as contrast agents and therapeutic drug delivery. Recently, curcumin, a bioactive compound, was incorporated into titanium oxide nanoparticles and conjugated with the MCP-1 antibody. The resulting nanoplatforms exhibited promising attributes as contrast agents for MRI and could be utilized for the early diagnosis of atherosclerosis [69].
In recent times, considerable interest is being laid upon using naturally derived molecules, mostly from plant sources, for therapeutic applications. Plant-based natural products are also observed for the synthesis of biogenic nanoparticles. Besides, plant-based compounds are also spatially incorporated into specific nanocarriers as effective therapeutics. In this context, plant-based compounds loaded into nanocarriers also gained considerable attention for the treatment of vascular diseases, including CVDs, as it is evident that inorganic nanoparticles are being actively used as contrast agents and therapeutic drug delivery. Recently, curcumin, a bioactive compound, was incorporated into titanium oxide nanoparticles and conjugated with the MCP-1 antibody. The resulting nanoplatforms exhibited promising attributes as contrast agents for MRI and could be utilized for the early diagnosis of atherosclerosis [33].
References
- Cervadoro, A.; Palomba, R.; Vergaro, G.; Cecchi, R.; Menichetti, L.; Decuzzi, P.; Emdin, M.; Luin, S. Targeting inflammation with nanosized drug delivery platforms in cardiovascular diseases: Immune cell modulation in atherosclerosis. Front. Bioeng. Biotechnol. 2018, 6, 177.
- Peng, R.; Ji, H.; Jin, L.; Lin, S.; Huang, Y.; Xu, K.; Yang, Q.; Sun, D.; Wu, W. Macrophage-based therapies for atherosclerosis management. J. Immunol. Res. 2020, 2020, 8131754.
- Talev, J.; Kanwar, J.R. Iron oxide nanoparticles as imaging and therapeutic agents for atherosclerosis. Semin. Thromb. Hemost. 2020, 46, 553–562.
- Goncalves, K.O.; da Silva, M.N.; Sichhieri, L.B.; de Oliveira Silva, F.R.; de Matos, R.A.; Courrol, L.C. Aminolevulinic acid with gold nanoparticles: A novel theranostic agent for atherosclerosis. Analyst 2015, 140, 1974.
- Karagkiozaki, V.; Logothetidis, S.; Pappa, A.M. Nanomedicine for atherosclerosis: Molecular imaging and treatment. J. Biomed. Nanotechnol. 2015, 11, 191–210.
- Caruthers, S.D.; Cyrus, T.; Winter, P.M.; Wickline, S.A.; Lanza, G.M. Anti-angiogenic perfluorocarbon nanoparticles for diagnosis and treatment of atherosclerosis. WIREs Nanomed. Nanobiotechnol. 2009, 1, 311–323.
- Basnet, R.; Khadka, S.; Wang, Y.; Gupta, R. Research progress on diagnosis and treatment of atherosclerosis. Sci. Lett. 2020, 8, 18–22.
- Bejarano, J.; Navarro-Marquez, M.; Morales-Zavala, F.; Morales, J.O.; Garcia-Carvajal, I.; Araya-Fuentes, E.; Flores, Y.; Verdejo, H.E.; Castro, P.F.; Lavandero, S.; et al. Nanoparticles for diagnosis and therapy of atherosclerosis and myocardial infarction: Evolution toward prospective theranostic approaches. Theranostics 2018, 8, 4710–4732.
- Chen, J.; Zhang, X.; Millican, R.; Creutzmann, J.E.; Martin, S.; Jun, H.W. High density lipoprotein mimicking nanoparticles for atherosclerosis. Nano Converg. 2020, 7, 6.
- Beldman, T.J.; Senders, M.L.; Alaarg, A.; Perez-Medina, C.; Tang, J.; Zhao, Y.; Fay, F.; Deichmöller, J.; Born, B.; Desclos, E.; et al. Hyaluronan nanoparticles selectively target plaque-associated macrophages and improve plaque stability in atherosclerosis. ACS Nano 2017, 11, 5785–5799.
- McCarthy, J.R.; Korngold, E.; Weissleder, R.; Jaffer, F.A. A light-activated theranostic nanoagent for targeted macrophage ablation in inflammatory atherosclerosis. Small 2010, 6, 2041–2049.
- Shon, S.-M.; Choi, Y.; Kim, J.-Y.; Lee, N.K.; Park, J.-Y.; Schellingerhout, D.; Kim, N.-E. Photodynamic therapy using a protease-mediated theranostic agent reduces Cathepsin-B activity in mouse atheromata in vivo. Arterioscler Thromb Vasc Biol. 2013, 33, 1360–1365.
- Qin, J.; Peng, Z.; Li, B.; Ye, K.; Zhang, Y.; Yuan, F.; Yang, X.; Huang, L.; Hu, J.; Lu, X. Gold nanorods as a theranostic platform for in vitro and in vivo imaging and photothermal therapy of inflammatory macrophages. Nanoscale 2015, 7, 13991–14001.
- Yeager, D.; Chen, Y.S.; Litovsky, S.; Emelianov, S. Intravascular photoacoustics for image-guidance and temperature monitoring during plasmonic photothermal therapy of atherosclerotic plaques: A feasibility study. Theranostics 2014, 4, 36–46.
- Kosuge, H.; Sherlock, S.P.; Kitagawa, T.; Dash, R.; Robinson, J.T.; Dai, H.; McConell, M.V. Near infrared imaging and photothermal ablation of vascular inflammation using single-walled carbon nanotubes. J. Am. Heart Assoc. 2012, 1, e002568.
- Lobatto, M.E.; Fayad, Z.A.; Silvera, S.; Vucic, E.; Calcagno, C.; Mani, V.; Dickson, S.D.; Nicolay, K.; Banciu, M.; Schiffelers, R.M.; et al. Multimodal clinical imaging to longitudinally assess a nanomedical anti-inflammatory treatment in experimental atherosclerosis. Mol. Pharm. 2010, 7, 2020–2029.
- Park, D.; Cho, Y.; Goh, S.H.; Choi, Y. Hyaluronic acid–polypyrrole nanoparticles as pH-responsive theranostics. Chem. Comm. 2014, 50, 15014–15017.
- Nandwana, V.; Ryoo, S.R.; Kanthala, S.; McMahon, K.M.; Rink, J.S.; Li, Y.; Venkatraman, S.S.; Thaxton, C.S.; Dravid, V.P. High-Density Lipoprotein-like Magnetic Nanostructures (HDL-MNS): Theranostic agents for cardiovascular disease. Chem. Mater. 2017, 29, 2276–2282.
- Bagalkot, V.; Badgeley, M.A.; Kampfrath, T.; Deiuliis, J.A.; Rajagopalan, S.; Maiseyeu, A. Hybrid nanoparticles improve targeting to inflammatory macrophages through phagocytic signals. J. Control. Release 2015, 217, 243–255.
- Oumzil, K.; Ramin, M.A.; Lorenzato, C.; Hemadou, A.; Laroche, J.; Jacobin-Valat, M.J.; Mornet, S.; Roy, C.E.; Kauss, T.; Gaudin, K.; et al. Solid lipid nanoparticles for image-guided therapy of atherosclerosis. Bioconjug. Chem. 2016, 27, 569–575.
- Zhang, Y.; Koradia, A.; Kamato, D.; Popat, A.; Little, P.J.; Ta, H.T. Treatment of atherosclerotic plaque: Perspectives on theranostics. J. Pharm. Pharmacol. 2019, 71, 1029–1043.
- Dai, T.; He, W.; Yao, C.; Ma, X.; Ren, W.; Mai, Y.; Wu, A. Applications of inorganic nanoparticles in the diagnosis and therapy of atherosclerosis. Biomater. Sci. 2020, 8, 3784.
- Zhu, X.; Wang, H.; Zheng, L.; Zhong, Z.; Li, X.; Zhao, J.; Kou, J.; Jiang, Y.; Zheng, X.; Liu, Z.; et al. Upconversion nanoparticle-mediated photodynamic therapy induces THP-1 macrophage apoptosis via ROS bursts and activation of the mitochondrial caspase pathway. Int. J. Nanomed. 2015, 10, 3719–3736.
- Han, X.B.; Li, H.X.; Jiang, Y.Q.; Wang, H.; Li, X.S.; Kou, J.Y.; Zheng, Y.H.; Liu, Z.N.; Li, H.; Li, J.; et al. Upconversion nanoparticle-mediated photodynamic therapy induces autophagy and cholesterol efflux of macrophage-derived foam cells via ROS generation. Cell Death Dis. 2017, 8, e2864.
- Kharlamov, A.N.; Zubarev, I.V.; Shishkina, E.V.; Shur, V.Y. Nanoparticles for treatment of atherosclerosis: Challenges of plasmonic photothernal therapy in translational studies. Future Cardiol. 2018, 14, 109–114.
- Herranz, F.; Salinas, B.; Groult, H.; Pellico, J.; Lechuga-Vieco, A.V.; Bhavesh, R.; Ruiz-Cabello, J. Superparamagnetic nanoparticles for atherosclerosis imaging. Nanomater. 2014, 4, 408–438.
- McCarthy, J.R.; Jaffer, F.A.; Weissleder, R. A macrophage-targeted theranostic nanoparticle for biomedical applications. Small 2006, 2, 983–987.
- Nakhlband, A.; Eskandani, M.; Omidi, Y.; Saeedi, N.; Ghaffari, S.; Barar, J.; Garjani, A. Combating atherosclerosis with targeted nanomedicines: Recent advances and future prospective. BioImpacts 2018, 8, 59–75.
- Shiozaki, A.A.; Senra, T.; Morikawa, A.T.; Deus, D.F.; Paladino-Filho, A.T.; Pinto, I.M.F.; Maranhao, R.C. Treatment of patients with aortic atherosclerotic disease with paclitaxel-associated lipid nanoparticles. Clinics 2016, 71, 435–439.
- Banik, B.; Surnar, B.; Askins, B.W.; Banerjee, M.; Dhar, S. Dual-targetedsynthetic nanoparticles for cardiovascular diseases. ACS Appl. Mater. Interface 2020, 12, 6852–6862.
- Mog, B.; Asase, C.; Chaplin, A.; Gao, H.; Rajagopalan, S.; Maiseyeu, A. Nano-antagonist alleviates inflammation and allows for MRI of atherosclerosis. Nanotheranostics 2019, 3, 342–355.
- Brusini, R.; Dormont, F.; Cailleau, C.; Nicolas, V.; Peramo, A.; Varna, M.; Couvreur, P. Squalene-based nanoparticles for the targeting of atherosclerotic lesions. Int. J. Pharm. 2020, 581, 119282.
- Sherin, S.; Balachandran, S.; Abraham, A. Curcumin incorporated titanium dioxide nanoparticles as MRI contrasting agents for the early diagnosis of atherosclerosis-rat model. Vet. Anim. Sci. 2020, 10, 100090.
