Metabolic reactions that occur at alkylamino moieties may provide insight into the roles of these moieties when they are parts of drug molecules that act at different receptors. N-dealkylation of N,N-dialkylamino moieties has been associated with retaining, attenuation or loss of pharmacologic activities of metabolites compared to their parent drugs. Further, N-dealkylation has resulted in clinically used drugs, activation of prodrugs, change of receptor selectivity, and providing potential for developing fully-fledged drugs.
- N-alkylamino moieties
- metabolic N-dealkylation
- metabolic N-oxidation
- pharmacologic activity
- physicochemical properties
1. Introduction
Alkylamino moieties, either open chain aliphatic (secondary or tertiary), or heterocyclic tertiary ones, are common in drug molecules of various pharmacological classes. Their basicity and polarity are essential for drug action. They are found in antidepressants, antihistamines, narcotic analgesics, local anesthetics, as well as other drug classes. The order of prevalence of the alky groups in alkylamino moieties is methyl > ethyl > isopropyl > tert-butyl > others. Methyl, ethyl, and isopropyl groups are usually found in drug molecules as tertiary N,N-dimethylamino, N,N-diethylamino, or N,N-diisopropylamino moieties, respectively. In the metabolism of drug molecules containing N,N-dimethylamino, N,N-diethylamino and N,N-diisopropylamino moieties, the alkyl groups are mostly removed sequentially to give secondary and primary amino groups. On the other hand, tert-butyl groups are usually less prone to metabolic oxidative dealkylation (Figure 1). Intrinsic secondary N-alkylamino moieties–mostly methylamino–are also encountered in some drug molecules. Another route of N-alkylamino moiety metabolism is N-oxygenation, which is specific to only tertiary N,N-dialkylamino moieties either open-chain aliphatic or heterocyclic [1].
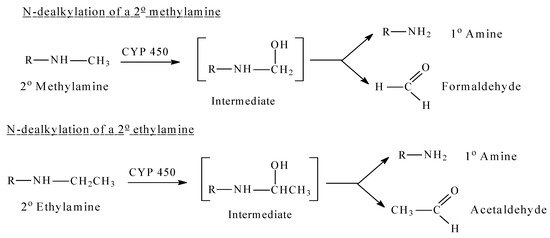
Figure 1. CYP 450 oxidative dealkylation of alkylamines.
Mechanistically, CYP450-catalyzed N-dealkylation involves as a first step the hydroxylation of the carbon atom of the alkyl group that is linked to the nitrogen atom (α-carbon atom). This hydroxylated metabolite is unstable. It breaks spontaneously into two molecules: the dealkylated metabolite (e.g., an amine), and an aldehyde (e.g., formaldehyde after demethylation, acetaldehyde after deethylation, etc.) [1]. The reaction is shown for methyl and ethyl secondary amines in Figure 1, which can be aliphatic or heterocyclic (or aromatic). Similarly, tertiary amines are dealkylated in a similar way by consecutive hydroxylation of the alkyl groups at the carbon that is linked to the nitrogen atom [1].
Depending on their class, alkylamino moieties interact with receptors or enzymes via hydrogen bonding, ion-dipole, ion-ion and van der Waals bindings as depicted in Figure 2 [2,3][2][3].
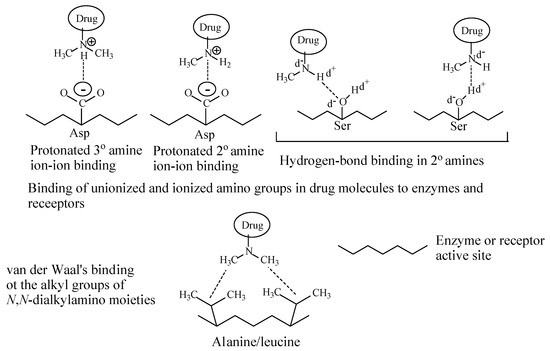
Figure 2. Alkylamino moiety binding to receptors.
The alkylamino-moieties-containing drugs cited in this review may be categorized according to the receptors upon which they act.
2. Neurotransmitter Reuptake Inhibitors
The drugs that contain alkylamino moieties in this class belong to four categories: (i) serotonin-norepinephrine reuptake inhibitors (SNRI), (ii) selective norepinephrine reuptake inhibitors (NRI), (iii) selective serotonin reuptake inhibitors (SRI), and (iv) serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRI).
2.1. Serotonin-Norepinephrine Reuptake Inhibitors (SNRI)
To this subclass belong the tricyclic antidepressants imipramine and amitriptyline, which are SNRI with preference for SRI, are respectively metabolized by N-demethylation to desmipramine and nortriptyline, (Figure 3 and Figure 4, respectively). Desipramine and nortriptyline have been developed into drugs of their own rights; they have preference for NR inhibition over SR inhibition.
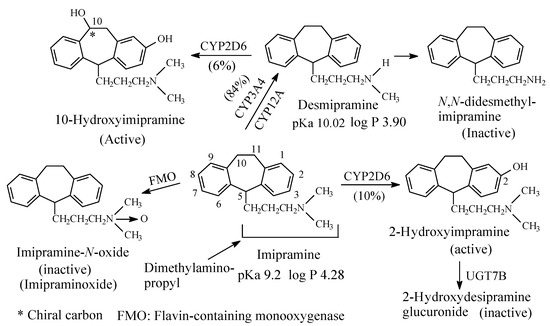
Figure 3. Metabolic pathways of imipramine.
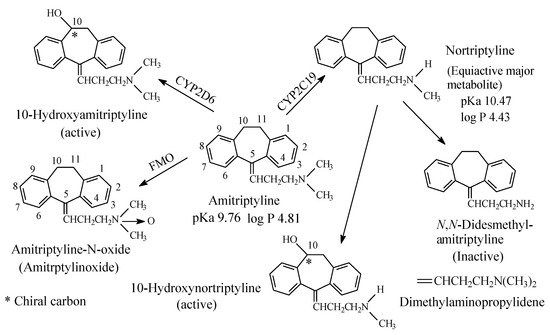
Figure 4. Metabolic pathways of amitriptyline.
2.1.1. Imipramine and Amitriptyline
Both imipramine and amitriptyline (Figure 3 and Figure 4, respectively) are aliphatic tertiary-amine tricyclic antidepressants. The two drugs are metabolized by N-demethylation to equiactive secondary-amine metabolites, desmipramine and nortriptyline, respectively, as shown in Figure 3 and Figure 4 [4,5,6,7,8][4][5][6][7][8]. The latter two drugs are further metabolized by N-demethylation to inactive primary amine metabolites [9]. Hydroxylation is also an important metabolic pathway of the four drugs (Figure 3 and Figure 4).
The four tricyclic antidepressant drugs are SNRI with imipramine and amitriptyline being more active as SRIs than NRIs and desmipramine and nortriptyline being more active as NRIs than SRIs [10]. Another metabolic pathway of imipramine and amitriptyline is N-oxygenation resulting in the formation of imipramine-N-oxide and amitriptyline-N-oxide, respectively. The two N-oxide metabolites have been developed as prodrugs of imipramine and amitriptyline since they (the N-oxide metabolites) are bioreduced in vivo to the tertiary amine parent drugs, imipramine and amitriptyline [11].
Reporting on comparison between imipramine and desmipramine, Rose and Westhead [12] found no difference between the two drugs in patients with primary depression regarding antidepressive effect or onset of action. According to the authors [12], reactive and endogenous depression responded equally well to either drug.
The pKa and log p values of the imipramine and desmipramine are given in Figure 3; the pKa and log p values of amitriptyline and nortriptyline are given in Figure 4.
2.1.2. Clomipramine
Clomipramine (Figure 5), a 3-chloro analog of imipramine, is a dibenzazepine-derivative tricyclic antidepressant (TCA). It contains a dimethylamino propyl moiety. Clomipramine acts on both noradrenergic and serotonergic transporters; however, with selectively for the serotonin transporter by inhibiting transporter action at presynaptic neuronal sites [13]. Inhibition of the serotonin transmitter by clomipramine is in contrast to its principal active metabolite, N-desmethylclomipramine (Figure 5), which principally acts as antagonist of noradrenergic transporter receptor [13,14][13][14]. The effectiveness of clomipramine, compared to other TCAs, in the management of obsessive-compulsive disorder (OCD) may be related to its relative specificity for serotonin reuptake system inhibition [14]. This observation may suggest that OCD might be caused, in part, by dysregulation of the serotonergic system. Further metabolic pathways of clomipramine include aromatic ring hydroxylation to active 8-hydroxyclomipramineand and N-oxygenation to clomipramine-N-oxide [15]. The pKa and log p values of clomipramine and N-desmethylclomipramine are given in Figure 5.
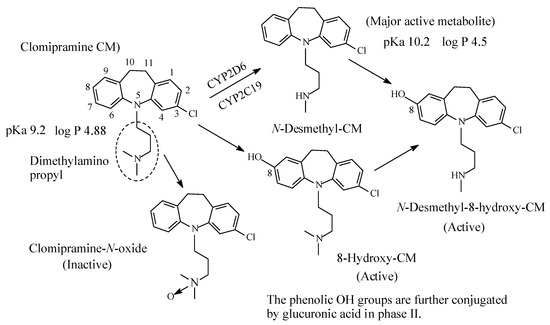
Figure 5. Metabolic pathways of clomipramine.
2.1.3. Venlafaxine
Venlafaxine (Figure 6) is an antidepressant drug that acts as both serotonin and norepinephrine reuptake inhibitor. It contains a dimethylaminomethyl moiety. The metabolic pathways of venlafaxine are depicted in Figure 6 [16,17,18,19][16][17][18][19]. While O-desmethylvenlafaxine is equiactive and equipotent with the parent drug as antidepressant and has been developed into a drug of its own right under the name of desvenlafaxine, N-desmethylvenlafaxine is devoid of antidepressant activity [20,21][20][21]. The pKa and log p values of venlafaxine and desvenlafaxine are given in Figure 6.
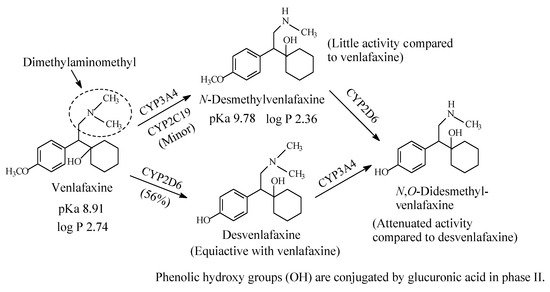
Figure 6. Major metabolic pathways of venlafaxine.
2.1.4. Doxepin
Doxepin (Figure 7) is a tricyclic antidepressant of the dibenzoxepine class. It contains a dimethylaminopropylidino moiety and exists in two geometric forms: E and Z in the ratio of 85:15, respectively [22]. The Z isomer is more active than the E isomer as antidepressant [23]. As far as the mechanism of action of doxepin is concerned, the E isomer is NRI while the Z isomer is SRI [24,25][24][25]. However, both isomers are metabolized by N-demethylation to give E-nordoxepin and Z-nordoxepin, which are active antidepressants and by N-oxidation to give E-doxepin-N-oxide and Z-doxepin-N-oxide, which are inactive as antidepressants [26]. The pKa and log p values of E-doxepin and E-N-nordoxepin are given in Figure 7.
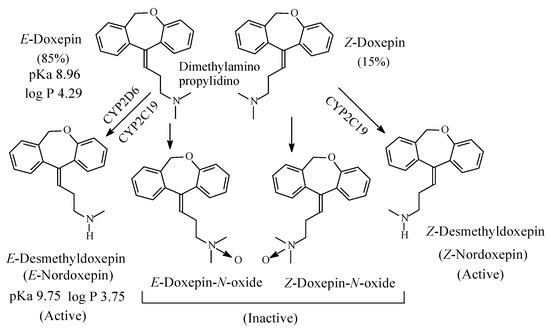
Figure 7. Metabolic pathways of doxepin.
2.2. Selective Norepinephrine Reuptake Inhibitors (NRIs)
2.2.1. Maprotiline
Maprotiline (Figure 8) is a tetracyclic antidepressant. It contains a secondary methylaminopropyl moiety. The mechanism of action of maprotiline involves selective norepinephrine neuronal reuptake inhibition. The metabolic pathways of maprotiline are depicted in Figure 8 with N-desmethylmaprotiline forming the major active metabolite [27,28,29,30,31][27][28][29][30][31]. The log p values of maprotiline and N-desmethylmaprotiline in addition to the pKa value of maprotiline are given in Figure 8. No value has been found for the pKa value of N-desmethylmaprotiline; however, it can be estimated to be higher than that of maprotiline.
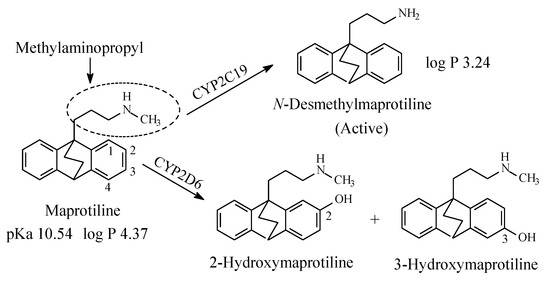
Figure 8. Major metabolic pathways of maprotiline.
2.2.2. Atomoxetine
Atomoxetine (Figure 9) is a selective NRI used to treat attention deficit hyperactivity disorder (ADHD). It contains a secondary ethylmethylamino moiety and is metabolized as per the pathways shown in Figure 9 [32,33,34,35,36,37,38][32][33][34][35][36][37][38]. While aromatic-ring hydroxylation does not affect the blockade of the NET and produces an equipotent metabolite to the parent drug [35], N-demethylation causes nearly 20-fold loss of pharmacologic activity with respect to atomoxetine [37]. The pKa and log p values of atomoxetine and 4-hyroxy-N-desmethylatomoxetine and the pKa value of N-desmethylatomoxetine are given in Figure 9. No log p value has been found for N-desmethylatomoxetine; however, it can be estimated to be lower than that of atomoxetine.
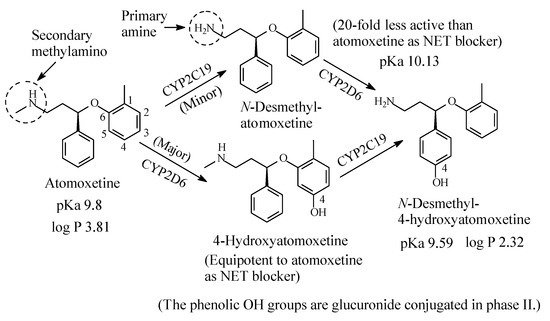
Figure 9. Metabolic pathways of atomoxetine.
References
- Rose, J.; Castagnoli, N., Jr. The metabolism of tertiary amines. Med. Res. Rev. 1983, 3, 73–88.
- Andrews, P.R.; Craik, D.J.; Martin, J.L. Functional group contributions to drug-receptor interactions. J. Med. Chem. 1984, 27, 1648–1657.
- Molecular Interactions. Cambridge MedChem Consulting. Available online: (accessed on 12 June 2020).
- Linden, R.; Antunes, M.V.; Ziulkoski, A.L. Determination of amitriptyline and its main metabolites in human plasma samples using HPLC-DAD application to the determination of metabolic ratios after single oral dose of amitriptyline. J. Braz. Chem. Soc. 2008, 19, 175–185.
- Imipramine Metabolism. Available online: (accessed on 13 June 2020).
- Sallee, F.R.; Pollock, B.G. Clinical pharmacokinetics of imipramine and desipramine. Clin. Pharmacokinet. 1990, 18, 346–364.
- Breyer-Ptaff, U. Amitriptyline-metabolism: The metabolic fate of amitriptyline, nortriptyline and amitriptylinoxide in man. Drug Metab. Rev. 2004, 36, 723–746.
- Amitriptyline Metabolism. Available online: (accessed on 13 June 2020).
- Williams, D.A. Antidepressants. In Foye’s Principles of Medicinal Chemistry, 7th ed.; Lemke, T.L., Williams, D.A., Roche, V.F., Zito, S.W., Eds.; Wolters Kluwer and Lippincott Williams and Wilkins: London, UK, 2013; p. 604. ISBN 978-81-8473-345-672-4.
- Maan, J.S.; Rosani, A.; Saadabadi, A. Desipramine. StatPearls. Available online: (accessed on 14 June 2020).
- Anzenbacher, P.; Zanger, U.M. (Eds.) Metabolism of Drugs and Other Xenobiotics; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 285–300. ISBN 978-3-527-64632-6.
- Rose, T.J.; Westhead, T. Comparison of desipramine and imipramine in depression. Am. J. Psychiatry 1964, 121, 496–498.
- Williams, D.A. Antidepressants. In Foye’s Principles of Medicinal Chemistry, 6th ed.; Chapter 21; Lemke, T.L., Williams, D.A., Roche, V.F., Zito, S.W., Eds.; Wolters Kluwer/Lippincott Williams & Wilkins: London, UK, 2008; pp. 547–600. ISBN 9780781768795 0781768799.
- Metabolism of Clomipramine. Available online: (accessed on 15 June 2020).
- Clomipramine Metabolism. Available online: (accessed on 15 June 2020).
- Sangkuhl, K.; Julia, C.; Sting, J.C.; Turpeinen, M.; Altman, R.B.; Kleina, T.E. PharmGKB summary: Venlafaxine pathway. Pharm. Genom. 2014, 24, 62–72.
- Hermann, M.; Hendset, M.; Fosaas, K.; Hjerpset, M.; Refsum, H. Serum concentrations of venlafaxine and its metabolites O-desmethylvenlafaxine and N-desmethylvenlafaxine in heterozygous carriers of the CYP2D6*3, *4 or *5 allele. Eur. J. Clin. Pharmacol. 2008, 64, 483–487.
- Metabolism of Venlafaxine. Available online: (accessed on 16 June 2020).
- Metabolism of Venlafaxine. Available online: (accessed on 18 July 2020).
- Sanchez, C.; Hyttel, J. Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell. Mol. Neurobiol. 1999, 19, 467–489.
- Kebamo, S.; Tesema, S.; Geleta, G.B. The Role of biotransformation in drug discovery and development. J. Drug Metab. Toxicol. 2015, 6, 5.
- Metabolism of Doxepin. Available online: (accessed on 20 June 2020).
- Metabolism of Doxepin. Available online: (accessed on 21 June 2020).
- Hobbs, D.C. Distribution and metabolism of doxepin. Biochem. Pharmacol. 1969, 18, 1941–1954.
- Shu, Y.Z.; Hubbard, J.W.; Cooper, J.K.; McKay, G.; Korchinski, E.D.; Kumar, R.; Midha, K.K. The identification of urinary metabolites of doxepin in patients. Drug Metab. Dispos. 1990, 18, 735–741.
- Center for Drug Evaluation and Research. Metabolism of doxepin; Application Number: 022036orig1s000. In Clinical Pharmacology and Biopharmaceutics Review(S); Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2009.
- Doxepin HCl. Available online: (accessed on 25 June 2020).
- Riess, W.; Fünfgeld, E.W.; Imhof, P.; Hürzeler, H.; Matussek, N.; Raschdorf, F.; Schmid, K. The pharmacokinetic properties of maprotiline (ludiomil) in man. J. Int. Med. Res. 1975, 3, 16–41.
- Metabolism of Maprotiline. Available online: (accessed on 28 June 2020).
- Metabolism of Maprotiline. Available online: (accessed on 28 June 2020).
- Brachtendorf, L.; Jetter, A.; Beckurts, A.T.; Hölscher, A.H.; Fuhr, U. Maprotiline metabolism CYP2D6 and CY)1A2: Cytochrome P450 enzymes contributing to demethylation of maprotiline in man. Basic Clin. Pharmacol. Toxicol. 2002, 90, 144–149.
- Metabolism of Atomoxetine. Available online: (accessed on 2 July 2020).
- Sauer, J.M., 1st; Ponsler, G.D.; Long, A.J.; Witcher, J.W.; Thomasson, H.R.; Desante, K.A. Disposition and metabolic fate of atomoxetine hydrochloride: The role of CYP2D6 in human disposition and metabolism. Drug Metab. Dispos. 2003, 31, 98–107.
- Metabolism of Atomoxetine. Available online: (accessed on 2 July 2020).
- Yu, G.; Li, G.-F.; Markowitz, J.S. Atomoxetine: A review of its pharmacokinetics and pharmacogenomics relative to drug disposition. J. Child. Adolesc. Psychol. Pharmacol. 2016, 26, 314–326.
- Dinh, J.C.; Pearce, R.E.; Haandel, L.V.; Gaedigk, A.; Leeder, J.S. Characterization of atomoxetine biotransformation and implications for development of PBPK models for dose individualization in children. Drug Metab. Dispos. 2016, 44, 1070–1079.
- Metabolism of Atomoxetine. Available online: (accessed on 2 July 2020).
- Laak, M.A.; Temmink, A.H.; Koeken, A.; van’t Veer, N.E.; van Hattum, P.R.M.; Cobbaert, C.M. Recognition of impaired atomoxetine metabolism because of low CYP2D6 activity. Pediatr. Neurol. 2010, 43, 159–162.
