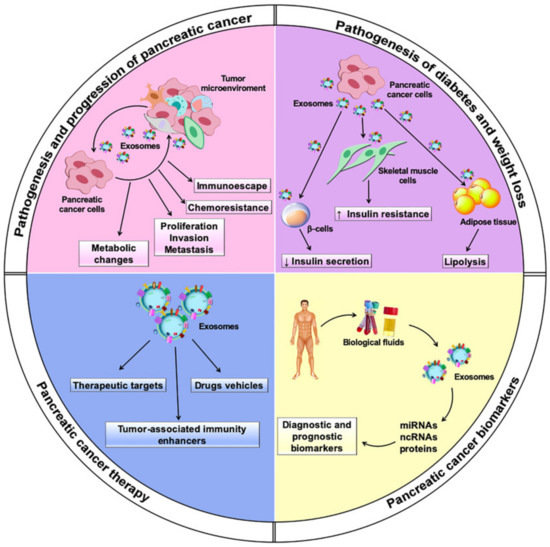
Multiple lines of evidence have showed that exosomes are actively involved in intercellular communication by transferring their cargos of bioactive molecules to recipient cells within the tumor microenvironment and systemically. Intriguingly, exosomes may exert both protumor and antitumor effects, supporting or hampering processes that play a role in the pathogenesis and progression of PC, including shifts in tumor metabolism, proliferation, invasion, metastasis, and chemoresistance. They also have a dual role in PC immunomodulation, exerting immunosuppressive or immune enhancement effects through several mechanisms. PC-derived exosomes also induce systemic metabolic alterations, leading to the onset of diabetes and weight loss. Moreover, exosomes have been described as promising diagnostic and prognostic biomarkers for PC. Their potential application in PC therapy as drug carriers and therapeutic targets is under investigation.
- pancreatic cancer
- extracellular vesicles
- biomarkers
- therapeutic targets
- drug vehicles
1. Introduction
Pancreatic cancer (PC) is considered an almost incurable disease, with 5-year survival barely reaching 10% [1]. Surgical resection is a potentially curative option for these patients, but approximately 80% of them present with advanced-stage unresectable disease at diagnosis [2]. Moreover, the 3-year recurrence rate exceeds 60% even when the most effective adjuvant chemotherapy regimens, such as mFOLFIRINOX, are used [3]. Therefore, for the majority of patients, chemotherapy represents the only available treatment, although results are largely unsatisfactory, with an overall survival of about 10 months [4]. Therapeutic strategies based on the use of natural compounds, novel synthetic molecules, or drug candidates for repurposing in oncology are also currently being explored, but these promising preclinical findings need additional evidence for translation into human therapy [5][6][7][8][9][10]. Unfortunately, PC appears to be a poorly immunogenic tumor characterized by the presence of a powerful immunosuppressive tumor microenvironment, which hinders substantial response to immunotherapy [11], whereas potential benefits of therapeutic strategies indicated in cases with specific molecular aberrations, such as defective mismatch repair or BRCA1/BRCA2 mutations, are limited to small subgroups of patients [12]. Thus, a better understanding of the mechanisms responsible for refractoriness to chemo- and immuno-therapy is necessary to design more effective therapeutic strategies for PC treatment.
The communication between cancer and non-neoplastic cells has been recognized to play a crucial role in carcinogenesis, chemoresistance, and immunosuppression [13]. Intercellular communication is an essential hallmark of organized cells in multicellular organisms and is mediated through direct cell–cell contact or the transfer of functional biomolecules. Over the last decade, multiple lines of evidence have proposed extracellular vesicles (EVs) as key signal transducers in intercellular communication. EVs are a heterogeneous population of vesicles classified according to their origin, size, and properties [13]. They deliver specific biological information to recipient cells and have emerged as crucial regulators of organized cell communities in several physiological and pathological processes, including cancer [13]. EV-mediated intercellular communication occurs through different biological mechanisms of EV uptake and content release. Uptake may occur through several routes, including endocytosis, macropinocytosis, and phagocytosis [14][15]. Receptor-mediated endocytosis involves specific ligands on cancer-derived EVs’ membrane, which bind surface receptors on recipient cells to activate intracellular signaling [14]. Caveola- or clathrin-dependent and lipid raft-dependent endocytosis, together with direct fusion, are other distinct mechanisms of internalization that are independent of EV ligands [14]. Many types of cells release EVs, including dendritic cells (DCs), B and T cells, neurons, fibroblasts, stem cells, and cancer cells [16]. Because of their substantial stability EVs circulate systemically and have been detected in biological body fluids (i.e., plasma, urine, saliva, breast milk) and pathological effusions [17]. Moreover, EVs cross different biological barriers, as indicated by the presence of glial/neuronal EVs in the cerebrospinal fluid, blood, tears, and urine [13].
Exosomes, which are the focus of this review, are a small subtype of EVs characterized by high stability in extracellular fluids and circulation. They contain a repertoire of bioactive molecules that can be transferred locally and systemically [17]. Profiling of exosome cargo is a strategy for their characterization and for determining their cellular origin [18]. Specific delivery to distant targets is ensured by their peculiar surface molecules, which determine tropism to distinct cells and tissues [13]. The cargo carried by exosomes can be functionally exchanged between tumor and non-tumor cells to support key processes in cancer, including growth, angiogenesis, invasion, and pre-metastatic niche formation [19] (Figure 1).
Figure 1. Multiple roles played by exosomes in pancreatic cancer.
2. Exosomes as Biomarkers for Diagnosis and Prognosis of Pancreatic Cancer
PC diagnosis is very often delayed by the lack of specific symptoms at early stages of the disease. Thus, most patients at diagnosis are already affected by locally advanced or metastatic disease, which is resistant to current treatments, resulting in a very poor prognosis [1]. Commonly used imaging techniques, such as computed tomography and endoscopic ultrasound, are unsatisfactory for PC screening [20]. Serum carbohydrate antigen (CA19-9) is the only FDA-approved biomarker for PC diagnosis, albeit with low sensitivity and specificity (70–90% and 68–91%, respectively) [16]. Hence, it is necessary to find novel robust tumor biomarkers for early PC diagnosis. Considering exosomes’ stability and their abundance in various biological fluids, exosomal miRNAs and proteins are amongthe candidates in the search for novel biomarkers (Table 1) [16].
Table 1. Exosomes as diagnostic and prognostic biomarkers for pancreatic cancer.
Biomarker Type | |
|---|---|
Exosomal Marker | |
Sample Size | |
Clinical Significance | |
Ref. | |
Diagnostic | ||
ZIP4 (serum) | ||
70 (24 PCs vs. 46 HCs) | ||
Discrimination between PCs and healthy controls | ||
| [ | 21 | ] |
miR-17-5p, miR-21 (serum) | ||
49 (22 PCs vs. 27 non-PCs/HCs) | ||
Discrimination between PC and non-PC patients (sensitivity 72.7% and specificity 92.6% for miR-17-5p); sensitivity 95.5% and specificity 81.5% for miR-21); high levels of miR-17-5p significantly correlate with advanced PCs | ||
| [ | 22 | ] |
miR-21, miR-155, miR-31, let-7a, miR-221, miR-181a, miR-935, miR-508 (plasma) | ||
60 (40 PCs/CPs vs. 20 HCs) | ||
Discrimination between PCs/CPs and healthy controls | ||
| [ | 23 | ] |
miR-10b, miR-21, miR-30c, miR-181a, miR-let7a (plasma) | ||
46 (29 PCs vs. 17 CPs/HCs) | ||
MicroRNA signature discriminating between PCs and CPs/healthy controls, sensitivity and specificity of | ||
100% for all biomarkers | ||
| [ | 24 | ] |
miR-10b (plasma) | ||
9 (3 PCs vs. 6 CPs/HCs) | ||
Discrimination between PCs and CPs/healthy controls | ||
| [ | 25 | ] |
miR-196a, miR-1246 (plasma) | ||
30 (15 PCs vs. 15 HCs) | ||
Discrimination between PCs and healthy controls | ||
| [ | 26 | ] |
miR-3940-5p/miR-8069 (urine) | ||
80 (43 PCs vs. 37 CPs/HCs) | ||
Discrimination between PCs and CPs/healthy controls; exosomal miRNA ratio higher in urine than in sera of PC patients | ||
| [ | 27 | ] |
Glypican-1 (GPC1) (serum) | ||
290 (190 PC vs. 100 HCs) | ||
Discrimination between PCs and healthy controls or benign pancreatic disease, sensitivity and specificity of | ||
100% | ||
| [ | 28 | ] |
Glypican-1 (GPC1) (serum) | ||
43 (22 PCs vs. 21 non-PCs/HCs) | ||
Discrimination between PCs, healthy controls or benign pancreatic disease, sensitivity 81% and specificity 52% | ||
| [ | 29 | ] |
PDAC | EV signature (EGFR, EPCAM, MUC1, GPC1, WNT2) (plasma) | |
43 (22 PCs vs. 21 non-PCs/HCs) | ||
Discrimination between PCs, healthy controls or benign pancreatic disease, sensitivity 86% and specificity 81% | ||
| [ | 29 | ] |
CKAP4 (serum) | ||
85 (47 PCs vs. 38 non-PCs/HCs) | ||
Discrimination between PC patients IHC+ for CKAP4 and PC patients IHC- for CKAP4, HCs or non-PC patients | ||
| [ | 30 | ] |
Prognostic | ||
miR-3607-3p (plasma) | ||
60 (40 PCs vs. 20 HCs) | ||
Low levels predict poor prognosis in PC patients | ||
| [ | 31 | ] |
miR-301a-3p (serum) | ||
62 (50 PCs vs. 12 HCs) | ||
High levels predict poor prognosis in PC patients | ||
| [ | 32 | ] |
Sox2ot (plasma) | ||
40 (20 PCs vs. 20 HCs) | ||
High levels correlate with TNM stage and poor overall survival in PC patients | ||
| [ | 33 | ] |
circ-PDE8A (plasma) | ||
113 (93 PCs vs. 20 non-PCs) | ||
High levels correlate with TNM stage and poor overall survival in PC patients | ||
| [ | 34 | ] |
circ-IARS (plasma) | ||
40 (20 metastatic PCs vs. 20 non-metastatic PCs) | ||
High levels correlate with TNM stage and overall survival in PC patients | ||
| [ | 35 | ] |
MIF (plasma) | ||
55 (40 metastatic/non-metastatic PCs vs. 15 HCs) | ||
High levels correlate with progression of disease post-diagnosis and prediction of liver metastasis | ||
| [ | 36 | ] |
miR-451a (plasma) | ||
70 (50 stage I/II PCs vs. 20 HCs) | ||
High levels predict recurrence and poor prognosis in PC patients | ||
| [ | 37 | ] |
Glypican-1 (GPC1) (serum) | ||
290 (190 PC vs. 100 HCs) | ||
High levels of GPC1 | + | exosomes correlate with tumor burden and reduced survival of PC patients |
| [ | 28 | ] |
Glypican-1 (GPC1) (serum) | ||
59 (27 PC vs. 32 non-PCs) | ||
High levels of GPC1 | + | exosomes correlate with tumor size |
| [ | 38 | ] |
c-Met, PDL-1 (serum) | ||
91 (55 PCs vs. 36 non-PCs) | ||
High levels after surgery predict poor survival for PC patients | ||
| [ | 39 | ] |
Abbreviations: Zinc transporter protein 4, ZIP4; Pancreatic cancers, PCs; Healthy controls, HCs; Chronic pancreatitis, CPs; Epidermal growth factor receptor, EGFR; Epithelial cell adhesion molecule, EPCAM; Mucin 1, MUC1; Wingless-type MMTV integration site family, member 2, WNT2; Cytoskeleton-associated protein 4, CKAP4; Immunohistochemistry, IHC; Macrophage migration inhibitory factor, MIF; Proto-oncogene mesenchymal-epithelial transition factor, c-Met; Programmed death-ligand 1, PD-L1.
References
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020.
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27.
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018.
- Goldstein, D.; El-Maraghi, R.H.; Hammel, P.; Heinemann, V.; Kunzmann, V.; Sastre, J.; Scheithauer, W.; Siena, S.; Tabernero, J.; Teixeira, L.; et al. nab-Paclitaxel Plus Gemcitabine for Metastatic Pancreatic Cancer: Long-Term Survival from a Phase III Trial. JNCI J. Natl. Cancer Inst. 2015, 107, dju413.
- Veschi, S.; De Lellis, L.; Florio, R.; Lanuti, P.; Massucci, A.; Tinari, N.; De Tursi, M.; di Sebastiano, P.; Marchisio, M.; Natoli, C.; et al. Effects of repurposed drug candidates nitroxoline and nelfinavir as single agents or in combination with erlotinib in pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2018, 37, 236.
- Florio, R.; Veschi, S.; di Giacomo, V.; Pagotto, S.; Carradori, S.; Verginelli, F.; Cirilli, R.; Casulli, A.; Grassadonia, A.; Tinari, N.; et al. The Benzimidazole-Based Anthelmintic Parbendazole: A Repurposed Drug Candidate That Synergizes with Gemcitabine in Pancreatic Cancer. Cancers. 2019, 11, 2042.
- Ammazzalorso, A.; De Lellis, L.; Florio, R.; Laghezza, A.; De Filippis, B.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Tortorella, P.; Veschi, S.; et al. Synthesis of novel benzothiazole amides: Evaluation of PPAR activity and anti-proliferative effects in paraganglioma, pancreatic and colorectal cancer cell lines. Bioorg. Med. Chem. Lett. 2019, 29, 2302–2306.
- De Filippis, B.; De Lellis, L.; Florio, R.; Ammazzalorso, A.; Amoia, P.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R.; Veschi, S.; et al. Synthesis and cytotoxic effects on pancreatic cancer cells of resveratrol analogs. Med. Chem. Res. 2019.
- Renz, B.; D’Haese, J.; Werner, J.; Westphalen, C.; Ilmer, M. Repurposing Established Compounds to Target Pancreatic Cancer Stem Cells (CSCs). Med. Sci. 2017, 5, 14.
- Pantziarka, P.; Verbaanderd, C.; Huys, I.; Bouche, G.; Meheus, L. Repurposing drugs in oncology: From candidate selection to clinical adoption. Semin. Cancer Biol. 2020.
- Torphy, R.J.; Zhu, Y.; Schulick, R.D. Immunotherapy for pancreatic cancer: Barriers and breakthroughs. Ann. Gastroenterol. Surg. 2018, 2, 274–281.
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA -Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019.
- Simeone, P.; Bologna, G.; Lanuti, P.; Pierdomenico, L.; Guagnano, M.T.; Pieragostino, D.; Del Boccio, P.; Vergara, D.; Marchisio, M.; Miscia, S.; et al. Extracellular Vesicles as Signaling Mediators and Disease Biomarkers across Biological Barriers. Int. J. Mol. Sci. 2020, 21, 2514.
- Möller, A.; Lobb, R.J. The evolving translational potential of small extracellular vesicles in cancer. Nat. Rev. Cancer 2020, 20, 697–709.
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977.
- Qiu, J.; Yang, G.; Feng, M.; Zheng, S.; Cao, Z.; You, L.; Zheng, L.; Zhang, T.; Zhao, Y. Extracellular vesicles as mediators of the progression and chemoresistance of pancreatic cancer and their potential clinical applications. Mol. Cancer 2018, 17, 2.
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Qasim, M.; Kim, J.-H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 307.
- Schey, K.L.; Luther, J.M.; Rose, K.L. Proteomics characterization of exosome cargo. Methods 2015, 87, 75–82.
- Guo, Y.; Ji, X.; Liu, J.; Fan, D.; Zhou, Q.; Chen, C.; Wang, W.; Wang, G.; Wang, H.; Yuan, W.; et al. Effects of exosomes on pre-metastatic niche formation in tumors. Mol. Cancer 2019, 18, 39.
- Guo, X.-Y.; Xiao, F.; Li, J.; Zhou, Y.-N.; Zhang, W.-J.; Sun, B.; Wang, G. Exosomes and pancreatic diseases: Status, challenges, and hopes. Int. J. Biol. Sci. 2019, 15, 1846–1860.
- Jin, H.; Liu, P.; Wu, Y.; Meng, X.; Wu, M.; Han, J.; Tan, X. Exosomal zinc transporter ZIP4 promotes cancer growth and is a novel diagnostic biomarker for pancreatic cancer. Cancer Sci. 2018, 109, 2946–2956.
- Que, R.; Ding, G.; Chen, J.; Cao, L. Analysis of serum exosomal microRNAs and clinicopathologic features of patients with pancreatic adenocarcinoma. World J. Surg. Oncol. 2013, 11, 219.
- Ali, S.; Dubaybo, H.; Brand, R.E. Differential Expression of Micrornas in Tissues and Plasma Co-exists as a Biomarker for Pancreatic Cancer. J. Cancer Sci. Ther. 2015, 7.
- Lai, X.; Wang, M.; McElyea, S.D.; Sherman, S.; House, M.; Korc, M. A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett. 2017, 393, 86–93.
- Joshi, G.K.; Deitz-McElyea, S.; Liyanage, T.; Lawrence, K.; Mali, S.; Sardar, R.; Korc, M. Label-Free Nanoplasmonic-Based Short Noncoding RNA Sensing at Attomolar Concentrations Allows for Quantitative and Highly Specific Assay of MicroRNA-10b in Biological Fluids and Circulating Exosomes. ACS Nano 2015, 9, 11075–11089.
- Xu, Y.-F.; Hannafon, B.N.; Zhao, Y.D.; Postier, R.G.; Ding, W.-Q. Plasma exosome miR-196a and miR-1246 are potential indicators of localized pancreatic cancer. Oncotarget 2017, 8, 77028–77040.
- Yoshizawa, N.; Sugimoto, K.; Tameda, M.; Inagaki, Y.; Ikejiri, M.; Inoue, H.; Usui, M.; Ito, M.; Takei, Y. miR-3940-5p/miR-8069 ratio in urine exosomes is a novel diagnostic biomarker for pancreatic ductal adenocarcinoma. Oncol. Lett. 2020.
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182.
- Yang, K.S.; Im, H.; Hong, S.; Pergolini, I.; Del Castillo, A.F.; Wang, R.; Clardy, S.; Huang, C.H.; Pille, C.; Ferrone, S.; et al. Multiparametric plasma EV profiling facilitates diagnosis of pancreatic malignancy. Sci. Transl. Med. 2017.
- Kimura, H.; Yamamoto, H.; Harada, T.; Fumoto, K.; Osugi, Y.; Sada, R.; Maehara, N.; Hikita, H.; Mori, S.; Eguchi, H.; et al. CKAP4, a DKK1 Receptor, is a Biomarker in Exosomes Derived from Pancreatic Cancer and a Molecular Target for Therapy. Clin. Cancer Res. 2019, 25, 1936–1947.
- Sun, H.; Shi, K.; Qi, K.; Kong, H.; Zhang, J.; Dai, S.; Ye, W.; Deng, T.; He, Q.; Zhou, M. Natural Killer Cell-Derived Exosomal miR-3607-3p Inhibits Pancreatic Cancer Progression by Targeting IL-26. Front. Immunol. 2019, 10.
- Wang, X.; Luo, G.; Zhang, K.; Cao, J.; Huang, C.; Jiang, T.; Liu, B.; Su, L.; Qiu, Z. Hypoxic Tumor-Derived Exosomal miR-301a Mediates M2 Macrophage Polarization via PTEN/PI3Kγ to Promote Pancreatic Cancer Metastasis. Cancer Res. 2018, 78, 4586–4598.
- Li, Z.; Jiang, P.; Li, J.; Peng, M.; Zhao, X.; Zhang, X.; Chen, K.; Zhang, Y.; Liu, H.; Gan, L.; et al. Tumor-derived exosomal lnc-Sox2ot promotes EMT and stemness by acting as a ceRNA in pancreatic ductal adenocarcinoma. Oncogene 2018, 37, 3822–3838.
- Li, Z.; Yanfang, W.; Li, J.; Jiang, P.; Peng, T.; Chen, K.; Zhao, X.; Zhang, Y.; Zhen, P.; Zhu, J.; et al. Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer. Cancer Lett. 2018, 432, 237–250.
- Li, J.; Li, Z.; Jiang, P.; Peng, M.; Zhang, X.; Chen, K.; Liu, H.; Bi, H.; Liu, X.; Li, X. Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis. J. Exp. Clin. Cancer Res. 2018, 37, 177.
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826.
- Takahasi, K.; Iinuma, H.; Wada, K.; Minezaki, S.; Kawamura, S.; Kainuma, M.; Ikeda, Y.; Shibuya, M.; Miura, F.; Sano, K. Usefulness of exosome-encapsulated microRNA-451a as a minimally invasive biomarker for prediction of recurrence and prognosis in pancreatic ductal adenocarcinoma. J. Hepatobiliary Pancreat. Sci. 2018, 25, 155–161.
- Frampton, A.E.; Prado, M.M.; López-Jiménez, E.; Fajardo-Puerta, A.B.; Jawad, Z.A.R.; Lawton, P.; Giovannetti, E.; Habib, N.A.; Castellano, L.; Stebbing, J.; et al. Glypican-1 is enriched in circulating-exosomes in pancreatic cancer and correlates with tumor burden. Oncotarget 2018.
- Lux, A.; Kahlert, C.; Grützmann, R.; Pilarsky, C. c-Met and PD-L1 on Circulating Exosomes as Diagnostic and Prognostic Markers for Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 3305.