Nosema locustae (Synonyms: Paranosema locustae, Antonospora locustae), a protozoan pathogen of locusts and grasshoppers, was developed as a biological control agent as early as the 1980s.
- locust
- grasshopper
- biological control
- Nosema locustae
- application
- epizootics
1. Introduction
Locust and grasshopper (L&G) outbreaks, often resulting in huge plagues, have been a serious threat to global food security since ancient times [1]. Traditional control of L&G consists mainly of the application of chemical pesticides, which often results in many side effects. These include toxic chemical residues on food, adverse health effects on humans and nontarget animals, and environmental pollution. One of the most promising alternatives to chemical pesticides is biological control. Although there are many natural enemies of L&G [2][3][4], only a few have been developed as biological control agents or potential agents, including the microsporidian Nosema locustae (synonym: Antonospora locustae, Paranosema locustae) and the fungus Metarhizium acridum, both of which have been quite widely used in the control of L&G.
N. locustae, a unicellular eukaryote (Figure 1) with an obligate intracellular lifestyle [5][6][7], was the first to be commercially developed for L&G control since its discovery and characterization of its potential as a biological control agent against these species [8][9][10]. Due to its slow action and various other constraints, N. locustae was considered to be of limited application [11][12]. However, as the only microsporidian agent for L&G control, N. locustae has been studied extensively [1][13][14][15][16][17]. In recent years, there has been a renewed interest in N. locustae, mainly due to work in China, where it is produced in large quantities and used extensively, and Argentina, where its long-term persistence appears to reduce the frequency and intensity of grasshopper outbreaks [18]. Recent advances in areas such as mass production, formulation, application, and epizootiology have further promoted the application of this pathogen. Numerous studies on its host spectra, molecular biology, and evolution have provided a solid fundamental basis for the use of N. locustae for L&G management.

Figure 1. Spores of N. locustae under scanning electron microscopy (photo by Long Zhang).
2. Host Spectrum
N. locustae was first identified in a laboratory population from the migratory locust Locusta migratoria (Linnaeus, 1758) by Elizabeth Canning in 1953 [19]. This pathogen occurs under natural conditions and has been found in various areas of the United States (Montana, Northern Dakota, Minnesota, Oregon, Wyoming, Colorado, Arizona, and Idaho), Canada (Saskatchewan, Ontario) and South America (western Pampas and northwestern Patagonia, Argentina), as well as Asia (Rajasthan and Vidarbha, India; Inner Mongolia, Hainan and Qinghai, China) and Africa (Karoo, South Africa) [20]. The highest level of infection was reported of about 5.5% in Melanoplus sanguinipes (Fabricius, 1798) in Idaho between 1963 and 1967 [21].
N. locustae has a wide host range restricted to orthopteran insects. Henry [22] in 1969 provided an initial list of 55 North American species that he knew to be susceptible. Brooks [15] published in 1988 a new list of worldwide susceptible orthopterans, expanding the host range to 95 species. The last review was done in 2005 by Lange [20], who arrived at a total of 121 species. Since then, various authors have been added to this list (Table 1), and we now reach a total of 144 susceptible orthopterans.
Table 1. List of orthopteran species infected by N. locustae but not included in Lange [20].
| Species | Inoculation Infection (Caging) | Field Trial Infection | References |
|---|---|---|---|
| Asia | |||
| Bryodemella holdereri (Krauss, 1901) | X | [23] | |
| Calliptamus italicus (Linnaeus, 1758) | X | [24] | |
| C. abbreviatus Ikonnikov, 1913 | X | [23] | |
| Ceracris kiangsu Tsai, 1929 | X | X | [25] |
| Chondracris rosea (De Geer, 1773) | X | X | [26] |
| Chorthippus dubius (Zubovski, 1898) | X | [27] | |
| C. brunneus (Thunberg, 1815) | X | [23] | |
| Damalacantha vacca (Fischer von Waldheim, 1846) | X | ||
| Deracanthella aranea (Fischer von Waldheim, 1833) | X | ||
| Dociostaurus kraussi (Ingenitskii, 1897) | X | [24] | |
| Fruhstorferiola tonkinensis (Willemse, 1921) | X | [28] | |
| Gampsocleis sedakovii (Fischer von Waldheim, 1846) | X | [23] | |
| Haplotropis brunneriana Saussure, 1888 | X | [24] | |
| Oedaleus decorus (Germar, 1825) | X | ||
| Arcyptera meridionalis Ikonnikov, 1911 | X | X | |
| Sphingonotus mongolicus Saussure, 1888 | X | [23] | |
| Africa | |||
| Acrotylus blondeli Saussure, 1884 | X | [29] | |
| Acrotylus patruelis (Herrich-Schäffer, 1838) | X | ||
| Aiolopus thalassinus (Fabricius, 1781) | X | ||
| Anacridium melanorhodon (Walker, 1870) | X | ||
| America | |||
| Amblytropidia australis Bruner, 1904 | X | [30] | |
| Dichroplus vittigerus (Blanchard, 1851) | X | ||
Although N. locustae has a broad host spectrum in Orthoptera, it is incapable of affecting non-orthopteran insects. It has been shown that the honey bee (Apis mellifera) and the lepidoterans Heliothis zea and Agrotis ipsilon were not susceptible [15]. Menapace et al. [31] reported that honey bees were not infected, even when fed high doses of spores. The American cockroach Periplaneta americana and the spider Butalus occidentalis were also found to be non-susceptible [32]. N. locustae has been demonstrated to be safe for vertebrates. Brooks [15] summarized the assessment tests on primary skin irritation, acute dermal toxicity, acute inhalation toxicity and pathogenicity, subacute oral toxicity, acute oral toxicity, acute pathogenicity, and possible hazards of N. locustae to vertebrates, including rabbits, guinea pigs, rainbow trout, giant toads, mallards, ring-necked pheasant, mice, and rats. No significant effects were observed. The toxicity of N. locustae at 20 million spores/mL to certain non-target organisms, such as Coturnix japonica, Apis mellifera, Bombyx mori, Daphnia magna, and Brachydanio rerio, was examined in China. The results showed that there was no risk to honey bees per os at the maximum exposure dose tested, no deaths in the five animal species tested in the contact toxicity experiments, and N. locustae was relatively safe for nontarget beneficial organisms in the environment [33].
3. Pathogenicity
N. locustae, as an obligate parasite, reproduces in host target cells. Its infection involves the polar tube of the spore to inject its plasma into the target cells [5]. It causes high mortality in L&G. The locust’s main target organ is the host’s adipose tissue (fat body) [9]. N. locustae penetrates fat body cells and produces meronts, sporonts, sporoblasts, and spores. In the migratory locust, Locusta migratoria migratorioides (Reiche & Fairmaire, 1849), the younger the nymphs, the more susceptible they are, but even newly emerged adults are still susceptible [9]. Studies by Tounou et al. [34] on the effects of N. locustae on the desert locust, Schistocerca gregaria (Forskål, 1775) and the Senegalese grasshopper, Oedaleus senegalensis (Krauss, 1877), showed that N. locustae has high pathogenicity on the young nymphal instars of these two species. The median survival time for first, second, third, fourth, and fifth nymphal instars was 6, 9, 10, 14, and 15 days, respectively, when locusts were inoculated with 1 × 107 spores on 10 g wheat bran in groups. Similar results were obtained with the Senegalese grasshopper using the same treatment method, with median survival times for instars 1, 3, and 5 being 5, 9, and 15 days, respectively. The median survival time therefore increased with the age of the locust and with decreasing inoculation doses. For instance, third-instar desert locust nymphs were inoculated in groups with 5.62 × 106 or 3.16 × 104 spores with a median survival time of 14 and 16 days, respectively. However, cumulative mortality increases with the increase of spore concentration and decreases with the increasing age of the nymphs. N. locustae caused high mortality in young desert locust nymphs. Mortality was 100% in first and second nymphal instars inoculated in groups with 1 × 107 spores of N. locustae, as well as in first nymphal instars with 1 × 106 spores. With third and fourth nymphal instars inoculated with 1 × 107 spores, cumulative mortality was above 90%. Similar results were obtained with the Senegalese grasshopper, which was inoculated with 1 × 107 spores: 100% mortality was observed in the first instar but 88.5% mortality in the third instar. In the fifth instar, mortality was only 66.3% in desert locust and 70% in the Senegalese grasshopper. These results suggest that the appropriate period for the application of N. locustae is mainly between the first and fourth instars, i.e., a possible application period of about 20–28 days, taking into account an average duration of 5–7 days per nymphal instar.
Inoculation of the 1st–5th nymphal instars of Chondracris rosea (De Geer, 1773) with N. locustae resulted in 78.2% to 100% mortality in field caging experiments by Liu and Chen [26]. The LC50 was approximately 3.88 × 105 spores/mL for the 1st–3rd instars and 3.98 × 106 spores/mL for the 4th–5th instars. In a caging experiment on a large forest site, the same authors observed that C. rosea mortality was greater than 91.1% when they sprayed N. locustae at a rate of 5 × 107 spores/mL. At 1 × 108 spores/mL, mortality reached 100% 25 days after treatment [26]. Chen et al. [25] conducted a laboratory experiment with five concentrations of N. locustae spores (1 × 104, 1 × 105, 1 × 106, 1 × 107, and 1 × 108 spores/mL) to treat early stages (1st–2nd instar) of the yellow-spined bamboo locust (Ceracris kiangsu). Mortalities were 16.6, 32.9, 29.2, 34.0, and 83.7%, respectively, increasing with spore concentrations. They also found that yellow-spined bamboo locust mortality was 85.1% after the application of a suspension of N. locustae spores at a concentration of 5 × 107 spores/mL in field trials in the forest. Zang et al. [35] reported that mortality of Oxya chinensis (Thunberg, 1815) was about 65.4–68.1% 30 days after the field treatment of third-instar nymphs sprayed with 1.5 × 1010, 2.25 × 1010 spores/ha, respectively. The infection rate in survivors was approximately 40%.
Concurrent use of N. locustae and Metarhizium spp. has shown additive effects on locusts and grasshoppers. When fifth-instar nymphs of S. gregaria were inoculated first with N. locustae at doses between 1 × 104 and 1 × 106 spores on wheat bran in groups, and then 10 days later with M. anisopliae at doses between 1 × 102 and 1 × 104 spores/nymph, the median survival times ranged from 3 to 9 days, and the shortest duration was only 3 days at doses of 1 × 106 spores of Nosema and 1 × 104 spores of Metarhizium [29]. When the locusts were inoculated in groups with N. locustae at doses of 1 × 105 and 1 × 106 spores, and Metarhizium at 1 × 103 and 1 × 104/nymph, the mortality was at 90% and 97% over 3 days, and both reached 100% 10 days after inoculation. Mortality was only 12.2% in the control, showing a synergistic effect between these two agents. Similar results were obtained with N. locustae directly mixed with Metarhizium spp., with the oriental migratory locust (L. migratoria manilensis Meyen, 1835). Mortality was higher with the mixture compared to treatments with each agent applied separately. The effects of a mixture of M. acridum and N. locustae on third nymphal instars under laboratory conditions showed that at a ratio of 1:1 (M. acridum at 3.90 × 106 spores/g locust body weight and N. locustae at 3.99 × 106 spores/g locust body weight), locust mortality was about 96.7% 24 days after inoculation, showing an additive effect of these two agents [36]. When 2nd–3rd-instar nymphs of L. migratoria were inoculated with 1 × 106 spores/nymph of N. locustae, and after 3, 6 and 9 days with Metarhizium at 1 × 107 conidia/mL, an additive effect was only observed in nymphs inoculated with Nosema and then 9 days later with Metarhizium [37]. After examining the stage of Nosema development on Days 3, 6, and 9 after inoculation, they found that it was not until the Nosema spore maturation stage that locusts were most susceptible to fungal infection.
Lv et al. [38] identified 4 defensins from migratory locust palp transcriptomes, named LmigDEF1 (78 amino acids), LmigDEF3 (78 amino acids), LmigDEF4 (69 amino acids), and LmigDEF5 (67 amino acids) with theoretical isoelectric points (pI)/molecular weights (Mw, kDa) of 6.48/8.29, 6.56/8.37, 8.23/7.19, and 8.27/6.93, respectively. The expression patterns of LmigDEF1, LmigDEF3, and LmigDEF5 in the fat body and salivary glands were examined by qRT-PCR after the locusts were inoculated with N. locustae. Results indicated that all three defensins varied over time in the fat body and salivary glands after Nosema infection, the transcript level of the LmDEFs being at their lowest in the fat body over the 10 days. This indicates that Nosema infection reduced the locust’s immune response via the defensins and may explain why the coordinated use of Nosema and Metarhizium results in higher locust mortality [29][36]. In particular, for about four days when Nosema were sporulating after inoculation, the locusts were more easily infected by the fungus due to the lower level of the three types of defensins in the fat body [37]. In addition, Chen et al. [39] demonstrated the key role of N. locustae sporulation in locust mortality. They identified a spore wall protein, AlocSWP2 from N. locustae, containing four cysteines. AlocSWP2 has been detected in the wall of mature spores, sporoblasts, and sporonts during sporulation in the host body by immunocytochemistry localization experiments. AlocSWP2 was detected in the fat body of infected locust only on Day 9 after inoculation using RT-PCR. The survival percentage of infected locusts that received a dsRNA injection of AlocSWP2 on Days 15, 16, and 17 after inoculation of Nosema spores was significantly higher than that of infected locusts without dsRNA treatment. Similarly, the number of spores in locusts infected with Nosema and treated with RNAi of AlocSWP2 was significantly lower than that in infected locusts without RNAi of this gene. This indicates that this N. locustae spore wall protein is involved in sporulation, contributing to host mortality.
L. migratoria migratorioides infected with N. locustae demonstrated reduced sustainable flight capacity [9]. Zhang et al. [40] confirmed this with the oriental migratory locust (L. migratoria manilensis). Flight capacity of infected and healthy adult locusts, 5 to 15 days after emergence, was determined using a flying mill for 18 h. On average, the flight distance of healthy versus infected locusts was 14,279 vs. 864 m; flight speed, 1.23 vs. 0.51 m/s, flight time, 3 vs. 0.33 h; maximum flight distance, 72,538 vs. 1544 m; and maximum sustained flight time, 6.7 h vs. 0.1 h. The decrease in flight capacity may be due to the fact that N. locustae destroyed the fat bodies reducing the supplement of glyceride and fat as energy resources. In L. migratoria manilensis infested with N. locustae, the glyceride content decreased rapidly, while lipase activity increased in both hemolymph and total fat [41].
Locusts and grasshoppers infected with N. locustae have reduced fertility [13][42]. Reduced vitellogenin was observed in fourth-instar nymphs of L. migratoria manilensis inoculated with N. locustae: vitellogenin levels in the fat body, hemolymph, and ovaries were very low compared to the control [43]. The maximum vitellogenin level in infected vs. healthy locusts, respectively, were 4.663 vs. 18.655 mg/mL in the fat body, 2.627 vs. 7.603 mg/mL in the hemolymph, and 4.927 vs. 73.367 mg/mL in the ovaries. This explains why the reproductive capacity of infected locusts is low.
The disease caused by N. locustae is transmitted vertically in eggs and egg pods [5]. Infected females have been reported to lay eggs containing spores of N. locustae [42]. Raina et al. [44] reported vegetative stages of N. locustae in the yolk of the oocyte and spores in the eggs of L. migratoria. When fourth-instar nymphs were inoculated with a dose of 1.5 × 106 spores, the infected parents laid eggs and the prevalence of infection was 100% in the next generation. The disease was vertically transmitted up to 14 generations, and mortality due to vertical transmission at times reached more than 90%. Parents of S. gregaria and O. senegalensis infected with N. locustae produced progeny with a 50% infection rate, indicating high vertical transmission in these species [34].
4. Mass Production and Products
The production of N. locustae spores is done in vivo. The rearing of infected hosts is the main process for mass production (Figure 2). Grasshoppers Melanoplus bivittatus (Say, 1825) have been used as hosts in the United States. Henry et al. [10] pointed out that several factors influence production yield. Cage size and the number of individuals in each cage, light in the cage, and time of harvest, as well as factors such as grasshopper species and sex, are important. The general process is as follows: grasshopper nymphs are reared to the 4th–5th instars, inoculated with N. locustae spore suspension, and then reared until they die. The cadavers are collected and crushed, screened, and centrifuged to obtain a high concentration of spores, which are stored between −10 and −20 °C. In the United States, after several improvements in mass production techniques, a yield of 1 × 109 to 3 × 109 spores per grasshopper has been achieved. However, the use of Melanoplus differentialis (Thomas, 1865) as a host resulted in up to 7.1 × 109 spores per individual [45]. The time of harvest is an important factor in influencing the spore yield; the further away from the inoculation date, the more spores are obtained [17].
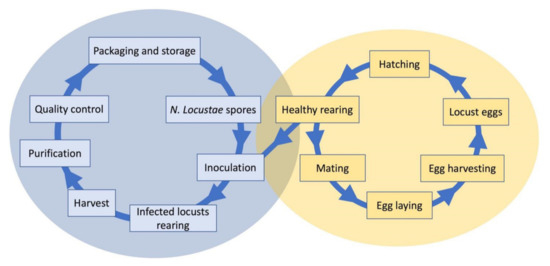
Figure 2. Mass producing process of N. locustae spores.
In China, L. migratoria manilensis has been selected as a host to produce spores of N. locustae. In this species, there is no diapause, and individuals can be used year-round. Several factors influence the efficiency of mass production, including the inoculation concentration, the stage of development for inoculation, and the harvesting time [46]. A higher concentration of spores in the inoculation suspension resulted in rapid locust death, and a lower concentration increased the time to harvest and reduced the number of spores. The best inoculation concentration was found to be 1 × 106 spores/mL, the stage for inoculation the fourth instar, and the harvest time about 30–40 days after inoculation. The average spores/individual yield was approximately 6 × 109, the highest being about 9.9 × 109. In China, several insectariums have been established for the mass production of N. locustae since the 1990s. Currently, the average spore yield per individual can reach 22 × 109 to 34 × 109. There are about 5–6 harvests per year, and the total yield can reach 2 × 1015 spores for each insectarium [17].
The products are usually formulated either as bran bait, aqueous suspension, or water-based suspension. They are prepared from high concentrations of spores stored at low temperature, diluted directly with water or mixed with wheat bran in an appropriate ratio. In China, a new water-based suspension has been developed. The spores are mixed with xanthan gum, sorbic acid, and other environmentally friendly additives. The suspension is more stable and homogeneous and can be stored for about one year at room temperature [47]. It is better adapted to the needs of users and should be widely applied. To date, several commercial products have been registered such as Nolo Bait™, Semaspore™, and Grasshopper Attack™ in the United States since the 1980s [12]. In China, Nosema locustae products for locust control have been developed since the 1990s and are currently distributed by Beijing JiaJing Biotechnology Ltd. (Beijing, China). They are based on a highly pathogenic strain (AL2008L-04) that significantly improves product efficacy, a high-yield production technology, and an aqueous suspension formulation that can be stored at room temperature [48].
References
- Zhang, L.; Lecoq, M.; Latchininsky, A.; Hunter, D. Locust and Grasshopper Management. Annu. Rev. Entomol. 2019, 64, 15–34.
- Greathead, D.J. Natural Enemies of Tropical Locusts and Grasshoppers: Their Impact and Potential as Biological Control Agents. In Biological Control of Locusts and Grasshoppers; Lomer, C.J., Prior, C., Eds.; CAB International: Wallingford, UK, 1992; pp. 105–122.
- Lomer, C.J.; Prior, C. Biological Control of Locusts and Grasshoppers; CAB International: Wallingford, UK, 1992.
- Goettel, M.S.; Johnson, D.L. (Eds.) Microbial Control of Grasshoppers and Locusts. Mem. Entomol. Soc. Can. 1997, 129, 400.
- Solter, L.F.; Becnel, J.J.; Oi, D.H. Microsporidian Entomopathogens. In Insect Pathology, 2nd ed.; Vega, F.E., Vega, H.K., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 221–263.
- Keeling, P.J.; Fast, N.M. Microsporidia: Biology and Evolution of Highly Reduced Intracellular Parasites. Annu. Rev. Microbiol. 2002, 56, 93–116.
- Keeling, P.J.; McFadden, G.I. Origins of Microsporidia. Trends Microbiol. 1998, 6, 19–23.
- Canning, E.U. The Life Cycle of Nosema locustae Canning in Locusta migratoria migratorioides (R. & F.) and its Infectivity to other Hosts. J. Inver. Pathol. 1962, 4, 237–247.
- Canning, E.U. The Pathologenicity of Nosema locustae Canning. J. Insect Pathol. 1962, 4, 248–256.
- Henry, J.; Tiahrt, K.; Oma, E. Importance of Timing, Spore Concentrations, and Levels of Spore Carrier in Applications of Nosema locustae (Microsporida: Nosematidae) for Control of Grasshoppers. J. Invertebr. Pathol. 1973, 21, 263–272.
- Lockwood, J.A.; Ewen, A.B. Biological Control of Rangeland Grasshoppers and Locusts. In The Bionomics of Grasshoppers, Katydids and their Kin; Gangwere, S.K., Muralirangan, M.C., Muralirangan, M., Eds.; CAB International: Wallingford, UK, 1997; pp. 421–442.
- Lockwood, J.A.; Bomar, C.R.; Ewen, A.B. The History of Biological Control with Nosema locustae: Lessons for Locust Management. Int. J. Trop. Insect Sci. 1999, 19, 333–350.
- Henry, J.E.; Oma, E.A. Pest Control by Nosema locustae, a Pathogen of Grasshoppers and Crickets. In Microbial Control of Pests and Plant Diseases (1970–1980); Burges, H.D., Ed.; Academic Press: New York, NY, USA, 1981; pp. 573–586.
- Henry, J.E. Natural and Applied Control of Insects by Protozoa. Annu. Rev. Entomol. 1981, 26, 49–73.
- Brooks, W.M. Entomogenous Protozoa. In CRC Handbook of Natural Pesticides; Apple Academic Press: Palm Bay, FL, USA, 2019; Volume 5, pp. 1–149.
- Lomer, C.J.; Bateman, R.P.; Johnson, D.L.; Langewald, J.; Thomas, M.B. Biological control of Locusts and Grasshoppers. Annu. Rev. Entomol. 2001, 46, 667–702.
- Johnson, D.L. Nosematidae and other Protozoa as Agents for Control of Grasshoppers and Locusts: Current Status and Prospects. Memoirs Entomol. Soc. Can. 1997, 129, 375–389.
- Lange, C.E.; Sokolova, Y.Y. The Development of the Microsporidium Paranosema (Nosema) locustae for Grasshopper Control: John Henry’s Innovation with Worldwide Lasting Impacts. Protistology 2017, 11, 170–174.
- Canning, E.U. A New Microsporidian, Nosema locustae n.sp., from the Fat Body of the African Migratory Locust, Locusta migratoria migratorioides R. & F. Parasitol. 1953, 43, 287–290.
- Lange, C.E. The Host and Geographical Range of the Grasshopper Pathogen Paranosema (Nosema) locustae Revisited. J. Orthoptera Res. 2005, 14, 137–141.
- Henry, J.E. Epizootiology of Infections by Nosema locustae Canning (Microsporidia: Nosematidae) in Grasshoppers. Acrida 1972, 1, 111.
- Henry, J.E. Extension of the Host Range of Nosema locustae in Orthoptera. Ann. Entomol. Soc. Am. 1969, 62, 452–453.
- Dong, Y. Preliminary Experiments on Application of Nosema locustae to Control Grasshoppers in Inner Mogolia Rangland. Master′s Thesis, Agricultural University, Beijing, China, 1989.
- Wang, L.; Yan, Y.; Dong, Y.; Lin, Z. Observations of a Microsporidian Parasite Nosema locustae Canning in the Oriental Migratory Locust (Locusta migratoria manilensis (Meyen)) and other Hosts from Inner Mogolia & Xinjiang. Acta Agric. Univ. Pekin. 1987, 13, 459–462.
- Chen, R.; Liu, Q.; Huang, H. Application of Insect Pathogenic Microbials to Control Ceracris kiangsu Tsai. Nat. Enem. Insects 2002, 24, 123–127.
- Liu, Q.; Chen, R. A Preliminary Study on Chondracris rosea Control by Inoculating Nosema locustae. Guangdong For. Sci. Technol. 1996, 12, 30–36.
- Ma, D.; Bai, S.; Li, Z. Studies on Sustainable Controlling Rangeland Grasshopper with Nosema locustae in Caidamu of Qinghai Province. J. Shandong Agric. Univ. Nat. Sci. 2005, 36, 199–205.
- Chen, Y.; Chen, Z.; Luo, R.; Chen, W.; Lu, S.; Li, Y. A Study on Application of Nosema locustae to Control Fruhstorferiola tonkinesis. In Proceedings of the 8th Conference of Chinese Entomology Society, Hebi, China, 11–13 October 2007.
- Tounou, A.-K.; Kooyman, C.; Douro-Kpindou, O.-K.; Poehling, H.-M. Interaction between Paranosema locustae and Metarhizium anisopliae var. acridum, two Pathogens of the Desert Locust, Schistocerca gregaria under Laboratory Conditions. J. Invertebr. Pathol. 2008, 97, 203–210.
- Lange, C.E.; Mariottini, Y.; Plischuk, S.; Cigliano, M.M. Naturalized, Newly-Associated Microsporidium Continues Causing Epizootics and Expanding its Host Range. Protistology 2020, 14, 32–37.
- Menapace, D.M.; Sackett, R.; Wilson, W.T. Adult Honey Bees are not Susceptible to Infection by Nosema locustae. J. Econ. Entomol. 1978, 71, 304–306.
- Krall, S.; Knausenberger, W. Efficiency and Environmental Impact for Biological Control of Nosema locustae on Grasshoppers in Cape Verde, a Synthesis Report; Deutsche Gesellschaft fur Technische Zusammenarbeit (GTZ): Eschborn, Germany, 1992; p. 16.
- Yuan, S.; Jiang, J.; Zhang, L.; Wu, S.; Liu, X.; An, X.; Li, G. Toxic Effects of Nosema locustae on Environmental Non-Target Bene-ficial Organisms. Mod. Agrochem. 2020, 19, 39–43.
- Tounou, A.; Kooyman, C.; Douro-Kpindou, O.; Gumedzoe, Y.; Poehlingn, H. Laboratory Assessment of the Potential of Paranosema locustae to Control Immature Stages of Schistocerca gregaria and Oedaleus senegalensis and Vertical Transmission of the Pathogen in Host Populations. Biocontrol Sci. Technol. 2011, 21, 605–617.
- Zang, J.; Cao, J.; Li, G.; Li, C.; Yan, Y. Biological Control of Oxya chinensis by using Nosema locustae. Chin. J. Boil. Control 2001, 17, 126–128. Available online: (accessed on 3 March 2021).
- Ding, X.; Zhang, L. Virulence of Metarhizium anisopliae and Nosema locustae Against the Nymphs of Locusta migratoria manilensis. J. Beijing Univ. Agric. 2009, 24, 9–14.
- Tokarev, Y.S.; Levchenko, M.V.; Naumov, A.M.; Senderskiy, I.V.; Lednev, G.R. Interactions of two Insect Pathogens, Paranosema locustae (Protista: Microsporidia) and Metarhizium acridum (Fungi: Hypocreales), during a Mixed Infection of Locusta migratoria (Insecta: Orthoptera) Nymphs. J. Invertebr. Pathol. 2011, 106, 336–338.
- Lv, M.; Mohamed, A.A.; Zhang, L.; Zhang, P.; Zhang, L. A Family of CSαβ Defensins and Defensin-Like Peptides from the Migratory Locust, Locusta migratoria, and Their Expression Dynamics during Mycosis and Nosemosis. PLoS ONE 2016, 11, e0161585.
- Chen, L.; Li, R.; You, Y.; Zhang, K.; Zhang, L. A Novel Spore Wall Protein from Antonospora locustae (Microsporidia: Nosematidae) Contributes to Sporulation. J. Eukaryot. Microbiol. 2017, 64, 779–791.
- Zhang, L.; Yan, Y.H.; Li, G.B.; Cao, Y.Z. The Effect of Nosema locustae Infection on Flying Ability of Locust (Locusta migratoria manilensis). Acta Agrestia Sin. 1995, 3, 324–327.
- Chen, J.; Shen, J.; Song, D.; Zhang, L.; Yan, Y. Effect of Nosema locustae on the Content of Fat in Locusta migratoria manilensis. Acta Entomol. Sin. 2000, 43, 109–113.
- Zhang, L. Epizootic and Transmission of Nosema locustae in the Populations of Grasshoppers and Locusts. Ph.D. Thesis, Beijing Agricultural University, Beijing, China, 1994.
- Chen, J.; Shen, J.; Song, D.; Zhang, L.; Yan, Y. Effect of Nosema locustae on the Content of Vitellogenin of Locusta migratoria manilensis. Acta Entomol. Sin. 2002, 45, 170–174.
- Raina, S.K.; Das, S.; Rai, M.M.; Khurad, A.M. Transovarial Transmission of Nosema locustae (Microsporida: Nosematidae) in the Migratory Locust Locusta migratoria migratorioides. Parasitol. Res. 1995, 81, 38–44.
- Henry, J.E. Effect of Grasshoppers Species, Cage Density, Light Intensity, and Method of Inoculation on Mass Production of Nosema locustae (Microsporida: Nosematiodae). J. Econ. Entomol. 1985, 78, 1245–1250.
- Wang, L.; Yu, X. Mass Production and Application of Nosema locustae Against Grasshoppers. Acta Agrestia Sin. 1994, 2, 49–54.
- Zhang, J. Research and Application of New Green Control Technology Extension Mode of Locusts in China. China agricultural university. Ph.D. Thesis, China Agricultural University, Beijing, China, 2015.
- Beijing JiaJing Biotecnology Ltd. Available online: (accessed on 26 March 2021).
