Rheumatoid arthritis (RA) is a widespread, chronic, autoimmune disorder affecting the joints, causing irreversible cartilage, synovium, and bone degradation. During the course of the disease, many immune and joint cells are activated, causing persistent inflammation. Immune cells including macrophages, lymphocytes, neutrophils, mast cells, natural killer cells, innate lymphoid cells, as well as synovial tissue cells, like fibroblast-like synoviocytes, chondrocytes, and osteoclasts secrete different pro-inflammatory factors, counting many cytokines, angiogenesis-stimulating molecules and others, exacerbating the ongoing condition. Curcumin is a natural polyphenol extracted from turmeric (Curcuma longa), with prominent anti-inflammatory and immunomodulatory effects. Various in vitro and in vivo studies have shown that curcumin can suppress the expression of inflammatory mediators and modulate immune cells, alleviating the course of RA, making it a promising, potential drug.
- rheumatoid arthritis
- curcumin
- immune cells
- immunomodulation
- autoimmune disease
1. Introduction
Rheumatoid arthritis (RA) is one of the most widespread chronic inflammatory diseases, affecting about 1% of the total world population [1,2[1][2][3],3], with yet unknown etiology. This autoimmune disorder is characterized by burdensome pain, swelling, and, usually, stiffness of symmetrical joints of hands, wrists, feet, and knees, greatly reducing mobility and overall comfort of life. During RA progression, persistent inflammation forces systematic changes causing irreversible cartilage, synovium, and bone degradation, finally deforming the whole joint structure, leading to loss of mobility and muscle atrophy [1,4,5][1][4][5]. This disease mostly affects joints; however, in approximately 40% of patients, it can also cause extraarticular manifestations of many sorts forming rheumatic nodules, causing lungs and blood vessel diseases or lead to anemia, peripheral neuropathy, and disorders in many different organs [4,6][4][6] (what is interesting, despite the fact that women are two to three times more likely to develop RA, they are less prone to develop extraarticular symptoms [2]). Moreover, rheumatoid arthritis features include loss of weight, fatigue, and fever, which, together with other manifestations, may lead to disability or even premature death [1,5,7][1][5][7].
During the course of the disease, a complicated network of relations is being established, self-perpetuating more and more aggressive inflammation. In sick patients, the joint maintains an unending conflict between anti- and proinflammatory factors with the second group’s dominance. After earlier initiation of an autoimmunologic reaction and inflammation establishment, leucocytes gradually infiltrate a joint due to the attractive properties of circulating chemokines, loosening of cartilage structures, and ongoing angiogenesis [8]. At the same time, fibroblast-like synoviocytes (FLS), which form a part of the synovium, change their functioning, hyperproliferating and releasing other disease-intensifying factors, including those involved in osteoclastogenesis. Due to the activity of activated chondrocytes and metalloproteinases released by synoviocytes, the joint cartilage is damaged. Degradation of mineral and non-mineral bone elements occurs mostly due to osteoclast- and synoviocyte-released cathepsin K activity [6,9,10][6][9][10].
2. Understanding the Pathogenesis of RA and the Effects of Curcumin
To better understand how RA affects human bodies, we need to know the synovium’s composition and function. The synovial membrane (synovium) is built by two layers composed of the intima and the underlying subintima layer. Subintima is mainly formed by vascularized connective tissue containing collagen fibers and evenly dispersed fibroblast- and macrophage-like synoviocytes (FLS and MLS). In a healthy synovium, this area normally has very few cells. Intima is a thin layer (1–2 cells-thick) composed mainly of FLS intercalated with MLS. The primary function of the synovium is to maintain joint homeostasis by regulating the synovial cavity influx and efflux. In this system, the intimal lining plays a crucial role—its loose fit allows the flow of essential substances and cells. Not only is the synovium responsible for regulating transport through the membrane, but its products are also vital for entire joint functioning. Fibroblast-like synoviocytes synthesize joint lubricants such as hyaluronic acid, oversee synovial fluid volume, regulate immunological processes, maintain extracellular matrix, secret hyaluronan, and clean intraarticular debris [11].
There are no direct causes that could lead to rheumatoid arthritis development because the disease itself seems to be of heterogeneous origin. RA appears to be more like a set of different (patient-dependent) but linked diseases leading to common outcomes. Possible proof for that statement may be found in the presence or absence of RA markers (i.e., specific antibodies) and differences in their levels, varying between patients at different stages of the disease [12]. The predisposition to RA development is about 60% hereditary. From 11% up to 37% of cases, it depends on the functioning of specific alleles of the human leukocyte antigen (HLA), which is involved in forming a major histocompatibility complex class 2 (MHCII) [1,6,13][1][6][13].
Besides possible genetic predispositions, a major role in RA development may be played by environmental factors such as diet, smoking, or contact with certain microbes (like Porphyromonas gingivalis), which can impact the patient’s immune system, mainly by increasing the number of autoantibodies.
Another important aspect is the role of epigenetic modifications in rheumatoid arthritis. DNA methylation, abnormal expression of non-coding RNAs, and cell type-specific histone modifications have been linked with RA [14,15][14][15]. It has been reported that hyperacetylation of histone H3 in the IL-6 promoter triggers the increase of IL-6 production by rheumatoid arthritis synovial fibroblasts (RASFs) [16]. Another study has shown that the extent of total histone H3 acetylation in peripheral blood mononuclear cells obtained from RA patients was increased compared to healthy controls [17]. Moreover, the balance between histone acetyltransferase (HAT) and histone deacetylase (HDAC) activity has been found to be significantly shifted towards histone acetylation in RA synovial tissue [18]. In 2015, a genome-wide study revealed the significant role of DNA methylation in lymphocyte populations obtained from RA patients [15,19][15][19]. Thus, the association between epigenetic modifications and pathogenesis of RA is indisputable.
Interestingly, many studies have described the potential role of curcumin as an epigenetic modifier. This potent herbal drug has been identified as an inhibitor of DNA methyltransferases (DNMTs), regulator of histone acetyltransferases (HATs), deacetylases (HDACs), and microRNAs, as well as a DNA binding agent [20]. Curcumin has been found to significantly reduce H3ac levels in the IL-6 promoter as well as IL-6 mRNA expression in rheumatoid arthritis synovial fibroblasts (RASFs) [16]. Even though the role of curcumin as an epigenetic modifier has been well documented in cancer, neurological disorders, and some inflammatory diseases [21], only a limited amount of in vitro and in vivo studies have been performed to establish the precise epigenetic regulatory effects of this herbal drug on RA models.
Although there is no cure available for rheumatoid arthritis, currently, the research is aimed at minimizing inflammation, pain, and joint damage, enhancing remission of symptoms, and improving the quality of life. Many studies have found that curcumin has a prominent effect on different immune cells and inflammatory mediators (Figure 1).
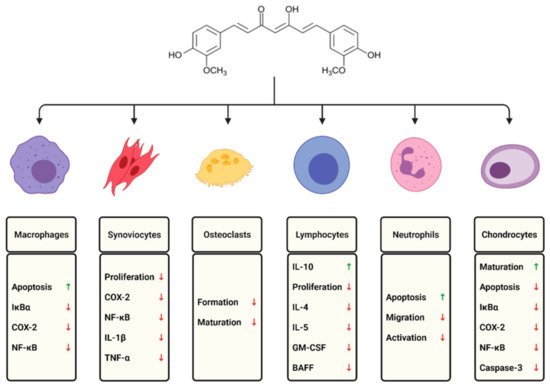
Figure 1.
The effects of curcumin on selected immune cells involved in the course of rheumatoid arthritis.
Curcumin is a polyphenolic substance naturally occurring in turmeric, especially in Curcuma Longa, with broad anti-inflammatory properties and proven positive effects in autoimmunological disease therapies, including RA. Curcumin is an antioxidant, which means it can efficiently reduce the level of reactive oxygen species (ROS), weaken redox signaling, and reduce inflammation [22]. In addition to having direct antioxidant properties, curcumin also blocks the activity of ROS-generating enzymes like lipoxygenase (LOX), cyclooxygenase (COX), xanthine dehydrogenase, and nitric oxide synthase (iNOS) [13,23,24][13][23][24]. Despite reducing ROS levels, curcumin also possesses numerous other properties that enable its usage as a potential therapeutic drug targeted against RA. Interesting insights into this matter are provided by recent studies, which found that this natural compound can suppress proinflammatory pathways related to the immune cells crucial in RA development. Therefore, curcumin’s daily consumption can decrease inflammation and oxidative stress, contributing to the immune system’s modulation and alleviating the rheumatoid arthritis course.
Besides beneficial properties, curcumin per se exhibits very low ADME (absorption, distribution, metabolism, excretion) scores, making it a challenging compound to use in potential therapies [25,26][25][26]. Several studies have shown that this polyphenol has a poor pharmacokinetic profile [27,28][27][28]. Under physiological conditions, curcumin is prone to undergo degradation via solvolysis and autoxidation, reduction by various enzymes and conjugation (glucuronidation or sulfonation), leading to loss of native values and being eliminated from the system [25,26,29][25][26][29]. Furthermore, curcumin’s low bioavailability and solubility in aqueous media, as well as susceptibility to photodegradation, makes it even more problematic [25]. To mitigate the abovementioned disadvantages, various vehicles such as formulations based on liposomes, phospholipid complexes, emulsions, or colloidal nanoparticles can be used [26]. A clinical trial evaluating an innovative and highly bioavailable formulation of curcumin showed significant analgesic and anti-inflammatory properties which relieved the symptoms of rheumatoid arthritis [30]. Nevertheless, novel formulations of curcumin and additional clinical trials on RA patients are still in dire need.
References
- Kumar, V.; Kanwar, J.R.; Verma, A.K. Rheumatoid arthritis: Basic pathophysiology and role of chitosan nanoparticles in therapy. In Advances and Avenues in the Development of Novel Carriers for Bioactives and Biological Agents; Singh, M.R., Singh, D., Kanwar, J.R., Chauhan, N.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 481–507. ISBN 978-0-12-819666-3.
- Turesson, C.; O’Fallon, W.M.; Crowson, C.S.; Gabriel, S.E.; Matteson, E.L. Extra-articular disease manifestations in rheumatoid arthritis: Incidence trends and risk factors over 46 years. Ann. Rheum. Dis. 2003, 62, 722–727.
- Sebbag, M.; Chapuy-Regaud, S.; Auger, I.; Petit-Texeira, E.; Clavel, C.; Nogueira, L.; Vincent, C.; Cornélis, F.; Roudier, J.; Serre, G. Clinical and pathophysiological significance of the autoimmune response to citrullinated proteins in rheumatoid arthritis. Jt. Bone Spine 2004, 71, 493–502.
- Waugh, A.; Grant, A. Ross and Wilson: Anatomy and Physiology in Health and Illness, 12th ed.; Elsevier: Amsterdam, The Netherland, 2013; pp. 297–301.
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., III; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2010, 62, 2569–2581.
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038.
- O’Dell, J.R. Rheumatoid Arthritis: The Clinical Picture. In Arthritis and Allied Conditions: A Textbook of Rheumatology; Isenberg, D.A., Madison, P.J., Woo, P., Klars, D., Breedveld, F.C., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 1165–1194.
- Elshabrawy, H.A.; Chen, Z.; Volin, M.V.; Ravella, S.; Virupannavar, S.; Shahrara, S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis 2015, 18, 433–448.
- Neumann, E.; Lefèvre, S.; Zimmermann, B.; Gay, S.; Müller-Ladner, U. Rheumatoid arthritis progression mediated by activated synovial fibroblasts. Trends Mol. Med. 2010, 16, 458–468.
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2009, 233, 233–255.
- Ouboussad, L.; Burska, A.N.; Melville, A.; Buch, M.H. Synovial Tissue Heterogeneity in Rheumatoid Arthritis and Changes With Biologic and Targeted Synthetic Therapies to Inform Stratified Therapy. Front. Med. 2019, 6, 45.
- Derksen, V.F.A.M.; Huizinga, T.W.J.; Van Der Woude, D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 437–446.
- Conigliaro, P.; Triggianese, P.; De Martino, E.; Fonti, G.L.; Chimenti, M.S.; Sunzini, F.; Viola, A.; Canofari, C.; Perricone, R. Challenges in the treatment of Rheumatoid Arthritis. Autoimmun. Rev. 2019, 18, 706–713.
- Nemtsova, M.V.; Zaletaev, D.V.; Bure, I.V.; Mikhaylenko, D.S.; Kuznetsova, E.B.; Alekseeva, E.A.; Beloukhova, M.I.; Deviatkin, A.A.; Lukashev, A.N.; Zamyatnin, A.A.J. Epigenetic Changes in the Pathogenesis of Rheumatoid Arthritis. Front. Genet. 2019, 10, 570.
- Mazzone, R.; Zwergel, C.; Artico, M.; Taurone, S.; Ralli, M.; Greco, A.; Mai, A. The emerging role of epigenetics in human autoimmune disorders. Clin. Epigenet. 2019, 11, 1–15.
- Wada, T.T.; Araki, Y.; Sato, K.; Aizaki, Y.; Yokota, K.; Kim, Y.T.; Oda, H.; Kurokawa, R.; Mimura, T. Aberrant histone acetylation contributes to elevated interleukin-6 production in rheumatoid arthritis synovial fibroblasts. Biochem. Biophys. Res. Commun. 2014, 444, 682–686.
- Li, Y.; Zhou, M.; Lv, X.; Song, L.; Zhang, D.; He, Y.; Wang, M.; Zhao, X.; Yuan, X.; Shi, G.; et al. Reduced Activity of HDAC3 and Increased Acetylation of Histones H3 in Peripheral Blood Mononuclear Cells of Patients with Rheumatoid Arthritis. J. Immunol. Res. 2018, 2018, 1–10.
- Lin, Y.-C.; Lin, Y.-C.; Wu, C.-C.; Huang, M.-Y.; Tsai, W.-C.; Hung, C.-H.; Kuo, P.-L. The immunomodulatory effects of TNF-α inhibitors on human Th17 cells via RORγt histone acetylation. Oncotarget 2016, 8, 7559–7571.
- Glossop, J.R.; Emes, R.D.; Nixon, N.B.; Packham, J.C.; Fryer, A.A.; Mattey, D.L.; Farrell, W.E. Genome-wide profiling in treatment-naive early rheumatoid arthritis reveals DNA methylome changes in T and B lymphocytes. Epigenomics 2016, 8, 209–224.
- Hassan, F.-U.; Rehman, M.S.-U.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an Alternative Epigenetic Modulator: Mechanism of Action and Potential Effects. Front. Genet. 2019, 10, 514.
- Boyanapalli, S.S.S.; Kong, A.-N.T. “Curcumin, the King of Spices”: Epigenetic Regulatory Mechanisms in the Prevention of Cancer, Neurological, and Inflammatory Diseases. Curr. Pharmacol. Rep. 2015, 1, 129–139.
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, Inflammation, and Chronic Diseases: How Are They Linked? Molecules 2015, 20, 9183–9213.
- Bright, J.J. Curcumin and Autoimmune Disease. Chem. Biol. Pteridines Folates 2007, 595, 425–451.
- Chandran, B.; Goel, A. A Randomized, Pilot Study to Assess the Efficacy and Safety of Curcumin in Patients with Active Rheumatoid Arthritis. Phytother. Res. 2012, 26, 1719–1725.
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin: Miniperspective. J. Med. Chem. 2017, 60, 1620–1637.
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930.
- Sharma, R.A.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and pharmacodynamics of curcumin. Adv. Exp. Med. Biol. 2007, 595, 453–470.
- Cheng, D.; Li, W.; Wang, L.; Lin, T.; Poiani, G.; Wassef, A.; Hudlikar, R.; Ondar, P.; Brunetti, L.; Kong, A.-N. Pharmacokinetics, Pharmacodynamics, and PKPD Modeling of Curcumin in Regulating Antioxidant and Epigenetic Gene Expression in Healthy Human Volunteers. Mol. Pharm. 2019, 16, 1881–1889.
- Ambreen, G.; Duse, L.; Tariq, I.; Ali, U.; Ali, S.; Pinnapireddy, S.R.; Bette, M.; Bakowsky, U.; Mandic, R. Sensitivity of Papilloma Virus-Associated Cell Lines to Photodynamic Therapy with Curcumin-Loaded Liposomes. Cancers 2020, 12, 3278.
- Amalraj, A.; Varma, K.; Jacob, J.; Divya, C.; Kunnumakkara, A.B.; Stohs, S.J.; Gopi, S. A Novel Highly Bioavailable Curcumin Formulation Improves Symptoms and Diagnostic Indicators in Rheumatoid Arthritis Patients: A Randomized, Double-Blind, Placebo-Controlled, Two-Dose, Three-Arm, and Parallel-Group Study. J. Med. Food 2017, 20, 1022–1030.
