Transition metal complexes with dppz-type ligands (dppz = dipyrido[3,2-a:2′,3′-c]phenazine) are extensively studied and attract a considerable amount of attention, becoming, from the very beginning and increasingly over time, a powerful tool for investigating the structure of the DNA helix. In particular, [Ru(bpy)2(dppz)]2+ and [Ru(phen)2(dppz)]2+ and their derivatives were extensively investigated as DNA light-switches.
- DNA
- luminescence
- light-switch
- sensors
- intercalation
1. Introduction
DNA has a vital role in life—cells use DNA to store and communicate all information necessary for the evolution of life. Its replication and transcription are the key mechanism underlying all biological functioning.
DNA is essentially polymorphic, although its most common structure is the anti-parallel double helix (B-DNA) [1]. However, its existence in several conformations, which are indispensable for its functioning and for the regulation of its functions, makes DNA a difficult target for small molecules. Therefore, research aimed at developing small molecules capable of binding to and reacting with DNA in all its forms is in continuous development. In fact, over the last 30 years, research led to the synthesis and study of molecules that bind to DNA as both diagnostic probes and therapeutic agents [2][3][4].
Among many others, inspired by the discovery and pharmaceutical activity of the effective anticancer agent cisplatin [5][6][7], the search for metal complexes capable of interacting with DNA is increasingly flourishing. In particular, attention is often directed towards a large family of transition metal complexes, as these species are able to combine structural elements, such as defined and modulable coordination geometries with appropriate ligand stability/lability, and distinctive electrochemical and photophysical properties.
On the one hand, the negative charge of the DNA surface implicitly allows one to consider electrostatic attraction to all cationic complexes as a possible interaction; on the other hand, one can also imagine that appropriately designed ligands (with extensive planar surfaces) might allow the metal complex to fit between the base pairs of double-stranded DNA in the B form (i.e., bind by intercalation).
This type of reasoning makes it possible to justify, for example, the different types of interaction with DNA offered by [Ru(bpy)3]2+ and [Ru(phen)3]2+ [8][9]. In fact, while the former binds only weakly to DNA, the interaction of [Ru(phen)3]2+ is much stronger, thanks to the presence of the phenanthroline ligand that allows for an at least partial intercalation into DNA base pairs, called semi-intercalation [10][11][12].
The initial interest was amplified by Barton′s research into the use of enantiopure forms of complexes like possible chiral probes for DNA [13][14][15]. Over time, it was realized that these were the photophysical and electrochemical properties of a given complex, that was achieved by appropriate ligand substitution, which actually expressed the great potential of these metal complexes as luminescent probes and photochemical reagents.
Furthermore, as well as allowing modulation of redox and implicitly photophysical properties, the design of appropriate ligands also provided a means of varying the character and strength of binding to nucleic acids.
In this review, rather than presenting a complete history of the development of metal complexes used as DNA binders, we focused on certain aspects of this class of compounds and, in particular, on the light-switch effect offered by the Ru(II) dppz derivatives [16].
2. DNA Structure
The classic 3D-model, proposed by Watson and Crick in 1953, describes the DNA (in B conformation) as two polynucleotide chains that coil around each other to form a right-handed double helix [17]. The two filaments of the DNA run in opposite directions to each other, so they are antiparallel, and according to the model, this is the reason such winding is present. In the center of the double helix there are nitrogenous bases, which keep the two chains bound by hydrophobic π-π interactions (which reduce the interaction with water molecules) and by the hydrogen bond between complementary bases, each of them belonging to a different chain. It is exclusively the interaction between a purine and pyrimidine derived base that grants the necessary distance so that a hydrogen bond can form—this can happen only by the A-T and G-C pairs.
Since hydrogen bonds are weak, it is relatively easy to break and form them again, like a zip, for example, due to the high temperature or mechanic interactions. As a consequence, in addition to the complementary bases, DNA molecules might be replicated such that all biological information contained within might be duplicated—such is the DNA replication [18].
It is quite well-known that G-C stability (three hydrogen bonds) is higher than that of A-T (two hydrogen bonds), therefore, the global stability of a DNA helix is related to the amount of G-C pairs, as well as the chain length.
The B form is the most common DNA conformation found within cells [19]. B-DNA winds up in a right-handed manner, see Scheme 1. There are approximately ten nitrogenous bases per turn, paired on the same plane and arranged perpendicularly to the DNA axis. The glycosidic bond between the 2-deoxyribose and the nucleobases shows anti-conformation. The stacking of the nucleobases leads to the formation of a major and a minor groove. In B-DNA, the major groove is 11.6 Å wide and the minor one is 6.0 Å wide—this difference leads to a different accessibility to the nitrogenous bases, depending on their position. Since on these grooves the nitrogenous bases are exposed to solvent, they might specifically interact with other species, without implicating modifications to the double helix. The majority of DNA-specific proteins interact with it right in one of the two grooves, especially into the major one because the sequence-specific recognition is greater in this groove.
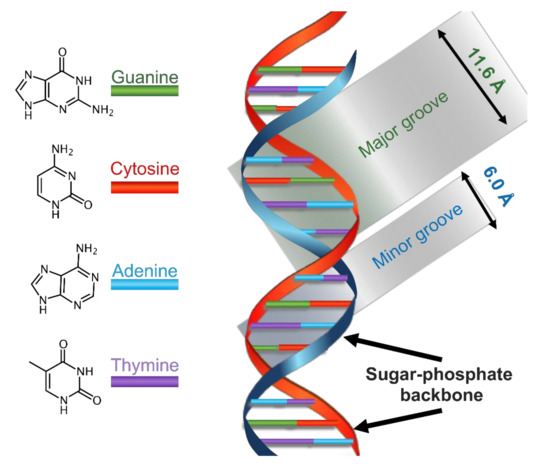
Scheme 1. Schematization of the main physical features of the structure of B-DNA.
References
- Rodley, G.A.; Scobie, R.S.; Bates, R.H.; Lewitt, R.M. A possible conformation for double-stranded polynucleotides. Proc. Natl. Acad. Sci. USA 1976, 73, 2959–2963.
- Zeglis, B.M.; Pierre, V.C.; Barton, J.K. Metallo-intercalators and metallo-insertors. Chem. Commun. 2007, 44, 4565–4579.
- Liu, H.K.; Sadler, P.J. Metal Complexes as DNA Intercalators. Acc. Chem. Res. 2011, 44, 349–359.
- Pages, B.J.; Ang, D.L.; Wright, E.P.; Aldrich-Wright, J.R. Metal complex interactions with DNA. Dalton Trans. 2015, 44, 3505–3526.
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386.
- Jung, Y.; Lippard, S.J. Direct Cellular Responses to Platinum-Induced DNA Damage. Chem. Rev. 2007, 107, 1387–1407.
- Florea, A.-M.; Büsselberg, D. Cisplatin as an Anti-Tumor Drug: Cellular Mechanisms of Activity, Drug Resistance and Induced Side Effects. Cancers 2011, 3, 1351–1371.
- Barton, J.K.; Goldberg, J.M.C.; Kumar, V.; Turro, N.J. Binding modes and base specificity of tris(phenanthroline)ruthenium(II) enantiomers with nucleic acids: Tuning the stereoselectivity. J. Am. Chem. Soc. 1986, 108, 2081–2088.
- Kelly, J.M.; Tossi, A.B.; McConnell, D.J.; OhUigin, C. A study of the interactions of some polypyridylruthenium(II) complexes with DNA using fluorescence spectroscopy, topoisomerisation and thermal denaturation. Nucleic Acids Res. 1985, 13, 6017–6034.
- Satyanarayana, S.; Dabrowiak, J.C.; Chaires, J.B. Tris(phenanthroline)ruthenium(II) enantiomer interactions with DNA: Mode and specificity of binding. Biochemistry 1993, 32, 2573–2584.
- Hiort, C.; Nordén, B.; Rodger, A. Enantiopreferential DNA binding of [ruthenium(II)(1,10-phenanthroline)3]2+ studied with linear and circular dichroism. J. Am. Chem. Soc. 1990, 112, 1971–1982.
- Lincoln, P.; Nordén, B. DNA Binding Geometries of Ruthenium(II) Complexes with 1,10-Phenanthroline and 2,2‘-Bipyridine Ligands Studied with Linear Dichroism Spectroscopy. Borderline Cases of Intercalation. J. Phys. Chem. B 1998, 102, 9583–9594.
- Barton, J.K. Tris(phenanthroline)metal complexes: Probes for DNA helicity. J. Biomol. Struct. Dyn. 1983, 1, 621–632.
- Barton, J.K.; Dannenberg, J.J.; Raphael, A.L. Enantiomeric selectivity in binding tris(phenanthroline)zinc(II) to DNA. J. Am. Chem. Soc. 1982, 104, 4967–4969.
- Nordén, B.; Tjerneld, F. Binding of inert metal complexes to deoxyribonucleic acid detected by linear dichroism. FEBS Lett. 1976, 67, 368–370.
- Li, G.; Sun, L.; Ji, L.; Chao, H. Ruthenium(II) complexes with dppz: From molecular photoswitch to biological applications. Dalton Trans. 2016, 45, 13261–13276.
- Watson, J.D.; Crick, F.H.C. Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid. Br. J. Nat. 1953, 171, 737–738.
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walters, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002.
- Leslie, A.G.; Arnott, S.; Chandrasekaran, R.; Ratliff, R.L. Polymorphism of DNA double helices. J. Mol. Biol. 1980, 143, 49–72.
