Collagen type I is the main organic constituent of the bone extracellular matrix and has been used for decades as scaffolding material in bone tissue engineering approaches when autografts are not feasible. Polymeric collagen can be easily isolated from various animal sources and can be processed in a great number of ways to manufacture biomaterials in the form of sponges, particles, or hydrogels, among others, for different applications. Despite its great biocompatibility and osteoconductivity, collagen type I also has some drawbacks, such as its high biodegradability, low mechanical strength, and lack of osteoinductive activity. Therefore, many attempts have been made to improve the collagen type I-based implants for bone tissue engineering.
- collagen
- bone tissue engineering
1. Collagen Type I in Bone Tissue Engineering
Collagen is the name used to designate a group of at least 29 different polymeric proteins that, together, are the most abundant protein component of the extracellular matrix (ECM) and represent around 20–30% of the weight of all body proteins in mammals [1]. All the collagen proteins are formed by a characteristic triple helix constituted by three out of all the genetically different pro-collagen polypeptide chains [2]. By far, the most abundant type of collagen is type I, which represents more than 90% of the organic mass of bone and is the major protein constituent of several other tissues like tendons, ligaments, cornea, or skin [2][3].
In bone, collagen type I is mainly produced by osteoblasts, which are also responsible for controlling the formation of hydroxyapatite from deposited calcium and phosphate salts. Osseous tissue is highly dynamic, and a continuous, strictly controlled turnover of its ECM occurs in healthy individuals by the osteoresorptive activity of osteoclasts and the osteogenic activity of osteoblasts. During the formation and the breakdown of the ECM, cells can interact with the exposed collagen fibers, which contain specific domains for interacting with cells, such as the well-known, integrin-binding motifs RGD [4] or DGEA [5]. Hence, when used as a scaffolding material in BTE, collagen not only acts as a physical support for cells to attach to and to grow on, but also influences cell behavior and fate through receptor-mediated interactions. These features make collagen type I a widely used biomaterial in tissue engineering applications, either to be used alone or to confer its biological properties to other scaffolds when used as composites [6][7].
Figure 1). Examples include the use of decalcified bone for filling bone defects in 1880, collagen isolated from rat tails with acetic acid and grafted into other animals, human cadavers or even living human patients, or collagen isolated from ox cornea and subcutaneously implanted in rabbits. In a more comprehensive study, Battista analyzed the histological reaction to implanting bovine bone-derived collagen (Collatissue A) into different locations, such as subcutaneously, in muscle, peritoneum, the nervous system, and bone [8]. This is one of the first reports on characterizing the cell response to collagen-based implants, observing an initial fibroblastic and monocyte infiltration followed by a gradual degradation until reaching a complete replacement by fibrous tissue.
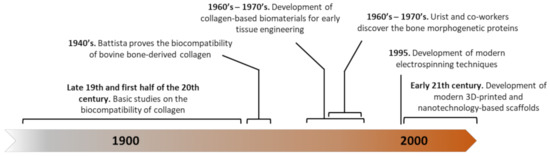
Figure 1. Timeline showing some of the major milestones in the development of collagen-based scaffolds for bone tissue engineering (BTE).
During the 1960s and 70s, several studies tested the grafting capacity, inflammatory response, degradation rates, vascularization, and other parameters in a great variety of collagen presentations, such as gels, sponges, meshes, or solutions in several models, such as rabbit eyes [9][10], stapedectomies [11], gynecological surgeries [12], or in skeletal defects in rabbit tibiae [13], ribs [14], and osteochondral defects [15], among others. All these studies together established that collagen-based biomaterials: (i) are biocompatible; (ii) have a tunable degradation rate; (iii) does elicit a mild inflammatory response; (iv) is invaded by the host’s cells; and (v) can become vascularized. These features made collagen progressively more popular for enhancing healing processes.
Although, at this point, it was already demonstrated that collagen type I-based biomaterials were suitable and biocompatible, they all failed in promoting successful bone healing when implanted into bone defects. In a series of works between 1965 and 1975, Marshall Urist and co-workers discovered that demineralized bone matrix was able to produce new bone after its subcutaneous or intramuscular implantation [16]. This demonstrated that isolated bone matrix contains osteoinductive biological cues that purified collagen type I lacks. Using different types of digestion, demineralization, and heat denaturation, these authors showed that the osteoinductive properties of bone matrix are related to a protein fraction different than the helical portion of collagen. They named this protein bone morphogenetic protein (BMP) and, with their discovery, opened an entire field in skeletal development and regeneration research [17][18][19][20]. Nowadays it is well known that BMP/transforming growth factor-β (TGF-β) signaling strongly regulates the ossification process during all its phases. Specifically, certain BMPs, such as BMP-2, -4, -6, -7, and -9 have a great osteoinductive effect in different settings, both in vitro and in vivo [21].
2. Forms of Collagen Type I Biomaterials for Bone Tissue Engineering
Table 1.
| Collagen Biomaterial Form | Combination/Modification | Biological Model | Main Results | References | ||
|---|---|---|---|---|---|---|
| Powders/particles | CBD-hFGF-2 | Mouse femur fracture | Increase of callus volume and bone mineral content | [22] | [33] | |
| CBD-rhBMP-2 | Vertebral laminar defects in rabbits | Greater bone regeneration. Signs of bone formation even without growth factor | [23] | [34] | ||
| ucMSC | Rabbit alveolar cleft model | Formation of a significant amount of new bone, higher percentage of bone trabeculae but no more mineral density | [24] | [35] | ||
| Fibers and tubes | - | Rat tibial nerve resection | Evidences of some degree of histological regeneration at surgical site | [25] | [36] | |
| UV irradiation crosslinking | Rat sciatic nerve section | Space of the tube is preserved and nerve repair was comparable to isograft treatment | [26] | [37] | ||
| BMSC | Mouse sciatic nerve section | Scaffolds loaded with cells induced better regeneration of peripheral nerve fibers | [27] | [38] | ||
| Electrospun in combination with HA | In vitro | cell viability and osteogenic differentiation assay | Good physicochemical properties and feasible manufacturing process. U2-OS cells remain viable and differentiate to osteoblast | [28] | [39] | |
| Electrospun with PLGA and HA nanorods | In vitro | cell viability and osteogenic differentiation assay | MC3T3-E1 cells proliferate on the scaffold. Osteogenic differentiation is evidenced by different markers | [29] | [40] | |
| Electrospun collagen containing catecholamines and Ca | 2+ | In vitro | human foetal osteoblasts viability and osteogenic differentiation | Good mechanical properties. Bio-inspired in situ chemical crosslinking and mineralization strategy. Osteogenic differentiation is evidenced by different markers | [30] | [41] |
| Electrospun in combination with chitosan | Rat full-thickness cranial defects | Composite had improved physicochemical properties and induced almost a total regeneration 8 weeks after implantation | [31] | [42] | ||
| Gels | HA particles and bone marrow cells | Rabbit posterolateral lumbar spine fusion model | Homogeneous new trabecular bone formation similar to autograft and BMP-HA group. | [32] | [43] | |
| Adipose-Derived stem cells + PLGA-β-TCP scaffold | Rabbit intramuscular ectopic bone formation assay | Composites showed new bone formation evidenced by radiography, histology and histomorphometric bone occupation analysis | [33] | [44] | ||
| rhBMP-2 | Rabbit tendon-bone interface injury model | Collagen-BMP-2 gel increased fusion rate between the bone tunnel and tendon | [34] | [45] | ||
| 3D printed in combination with HA | In vitro | viability and proliferation of Vero cells | Superior control over scaffold morphology and porosity. Supernatants of these gels incubated in medium were not cytotoxic | [35] | [46] | |
| 3D printed collagen containing rod-like nano-HA | - | Highly controlled 3D printed mesh-like structures with a homogeneous HA distribution. | [36] | [47] | ||
| 3D printed in combination with decellularized extracellular matrix and silk fibroin | In vitro | MC3T3 viability and osteogenic differentiation | Feasible hybrid 3D printing method. Cell proliferation and osteogenic differentiation was higher in comparison with only-collagen controls. | [37] | [48] | |
| Sponges | PGA + AD-MSC | Rabbit calvarial bone defect | Significant improvement of bone formation by CT scan imaging analysis induced by scaffolds with or without cells. | [38] | [49] | |
| Bone marrow mononuclear cells + Nano- Hydroxyapatite + platelet-rich fibrin | Human patients with unilateral alveolar cleft defects | Patients exhibited less donor site complications, faster and better soft tissue healing, less postoperative pain and a higher rate of complete alveolar bone union. | [39] | [50] | ||
| Precultured system of mesenchymal stem cells + mechano-chemical induction | Rat Achilles tendon repair model | Improved tenogenic differentiation | in vitro | . In vivo improvements in Achilles functional index, Young’s modulus and histology score | [40] | [51] |
| Umbilical cord mesenchymal stem cell-derived nanovesicles + rhBMP-2 | Nude mouse calvarial defect model | Micro-CT imaging analysis evidenced increased bone volume and number of trabeculae. Histology revealed increased number of vessel structures. | [41] | [52] |
2.1. Powders/Particles
One of the most explored forms of collagen in BTE is demineralized bone matrix (DBM) in its powder form. DBM powder is obtained when autologous or allogeneic bone is processed to yield a partially purified bone matrix, devoid of fat and mineral content, which is pulverized until converted into fine grains. A successful application of this type of collagen biomaterial has been in maxillary sinus floor elevation. After the loss of teeth in the maxillary region, the surrounding alveolar bone is markedly resorbed, creating a gap that makes the implantation of tooth implants extremely difficult. In these cases, DBM powders have been used to fill the created gap and to promote the formation of new bone, allowing the implantation of new dental pieces [42][43]. With a similar rationale, purified collagen powders have been used combined with other polymeric and non-polymeric materials as well as with growth factors. Saito and co-workers produced an injectable collagen powder combined with a chimeric, collagen-targeted, recombinant fibroblast growth factor-2 (CBD-FGF-2). These authors demonstrated that their system was able to increase callus volume and bone mineral content in a femoral fracture model in mice [22]. In a more recent study, bovine collagen powder was combined with BMP-2 to successfully enhance bone formation when implanted into vertebral laminar defects created in rabbits [23].
Collagen powder has also been used for increasing the effectiveness of cell therapy strategies. In a recent study, collagen powder was used as a scaffold for administering human umbilical cord-derived mesenchymal stem cells (huMSCs) in an alveolar cleft model in rabbits. The authors reported significantly better bone repair than when implanting the collagen without cells and, when focusing on certain parameters, even than when implanting collagen with BMP-2 [24]. Although they did not include a control group, implanting only cells without the collagen vehicle, it is somewhat predictable that collagen was acting as a scaffold on which cells were retained and induced to differentiate and, thus, increasing osteogenesis.
2.2. Fibers and Tubes
It is technically feasible to cast atelocollagen solutions in or around inert molds with a great variety of shapes to manufacture different types of biomaterials. Collagen tubes have been formed around silicone rods with the aim of improving nerve regeneration in a rat tibial nerve resection model [25]. Although collagen tubulization did not significantly enhance nerve regeneration, the tubulized repairs most closely resembled unoperated nerves. A later study showed that crosslinking by UV irradiation of collagen tubes promoted more cellular activity and regeneration compared to tubes that had been chemically crosslinked with glutaraldehyde [26]. When combined with bone marrow-derived mesenchymal stem cells (BM-MSCs), collagen tubes supported better regeneration of peripheral nerve fibers across a 3-mm nerve gap, thus demonstrating that these tubes could be used as regenerative guidance in cell therapy strategies [27].
For obvious anatomical reasons, tubular biomaterials are not frequently used in BTE in a direct way. However, more complex techniques, such as electrospinning manufacturing, are inspired in the fabrication of continuous fibers ranging between the nano- and the micro-scale, in a great variety of forms. This technique, which started to gain popularity for the manufacturing of materials for biomedical applications in the early 2000s, allows the production of polymer fibers with a diameter from 3 nm to more than 5 µm using a relatively simple and inexpensive setup. Although the disposition of the fibers is not finely controlled, highly porous membranes can be obtained. The most typical polymeric materials used for electrospinning are synthetic ones, like poly(L-lactic acid) (PLLA), poly(glycolic acid) (PGA), polycaprolactone (PCL), poly(ethylene oxide) (PEO), and poly(vinyl alcohol) (PVA). However, even though care must be taken to avoid protein denaturation during the process, polymers of natural origin can also be used, such as gelatin, silk fibroin, chitosan, and collagen. The theoretical and technical principles of this technique focused on tissue engineering applications and, specifically, on BTE have been extensively reviewed before [44][45].
Several studies have shown a successful production of polymeric collagen-based scaffolds using electrospinning. Collagen-HA membranes [28], alternate dual meshes of PLGA with a collagen-HA dispersion [29], and collagen-polycatecholamines-CaCO
3 scaffolds [30] displayed very good mechanical properties and low cytotoxicity, and sustained the osteogenic differentiation of different cells types in vitro. More recently, Guo and co-workers showed very promising results using a collagen-chitosan nanofibrous membrane to achieve almost total closure of full-thickness cranial bone defects in rats 8 weeks after implantation [31].
2.3. Gels
Hydrogels are very interesting materials, as they have a great ability to absorb water and allow the diffusion of cells and molecules. Since some of their physicochemical properties can be quite easily tailored during their manufacturing process by modifying their composition, crosslinking conditions, or synthetizing technique, they are highly versatile [46]. Although their poor biomechanical properties do not fulfill the requirements that could be considered ideal for BTE, hydrogels have the advantage of being easy to make in quantities and forms that can adapt to the specific needs of the surgeon.
A simple but interesting collagen format are injectable gels, which can be useful for physicians to fill bone defects using less invasive surgical techniques. Several models have been used to test fluid collagen biomaterials and compare these approaches with bone autografts. Minamide and co-workers analyzed collagen gels as cell carriers in vertebral arthrodesis. Therefore, they cultured bone marrow cells in a collagen type I gel for one week and used the whole in a rabbit spinal fusion model. They showed that this combination improved the success rate of fusions and led to the production of new bone tissue of better quality than autografts or the collagen gel without cells [32].
Collagen gels are also very suitable to be used as part of composite biomaterials to improve the biological or physicochemical properties of other polymeric or non-polymeric materials. As examples, collagen gels have been used to load adipose-derived mesenchymal stem cells into a porous poly(lactic-co-glycolic)acid tricalcium phosphate (PLGA-β-TCP) scaffold [33]. These authors showed that ectopic implantation of their composites in rabbits led to an increased and very homogenous calcified cartilage and bone formation compared to when the cells were delivered directly onto the PLGA-β-TCP scaffold without the collagen gel. This format has also been used as a BMP-2 carrier for its delivery into a tendon-bone interface injury model in rabbits [34]. Since collagen has some tendency to retain BMP-2, in vitro experiments showed that these gels were able to retain up to 50% of the loaded BMP-2 after 5 days, while slowly releasing the remaining growth factor for over 28 days. A single direct injection of this BMP-2-loaded gel induced the formation of fibrocartilage and new bone at the tendon-bone interface at 6 weeks after surgery.
In even more recent approaches, collagen gels have been tested as bioinks for 3D printing applications [47]. This technology allows printing of highly specific and detailed architectures and has been used for fabricating various tissue-like geometries, including collagen/hydroxyapatite composites [35], mesh-like structures of collagen containing hydroxyapatite nanorods [36] or even more complex scaffolds consisting of collagen, decellularized extracellular matrix and silk fibroin [37]. All of these scaffolds were demonstrated to be highly tunable, to have appropriate physicochemical properties, and to be biocompatible when tested in vitro.
2.4. Sponges
2.4. Sponges
Collagen sponges are, by far, the most widespread and tested form of collagen scaffold. They are usually manufactured by casting an aqueous collagen solution or gel into a mold and then freeze-dried. Changes in freezing regime, vacuum conditions, type of suspension, and presence of different additives or porogens can completely modify the porous structure, interconnectivity, and architecture of the resulting collagen sponge [48][49]. Even though these types of scaffolds have been extensively studied, clinically tested and some of them even globally commercialized, new technology is still being applied nowadays to increase the regenerative potential of collagen sponges. For example, the mechanical strength of a collagen sponge can be increased by adding synthetic polymers during its fabrication. In this line, poly (glycolic acid) fibers were added to collagen type I to produce collagen/PGA discs that significantly enhanced bone healing in calvarial defects in rabbits, even without the addition of growth factors or cells [38].
In more complex models, collagen sponges have been used as basic scaffolds for housing multifactorial elements. In this sense, Al-Ahmady and co-workers designed a collagen scaffold combined with platelet-rich fibrin and nanohydroxyapatite seeded with autologous bone marrow mononuclear cells for bone regeneration in a small clinical study with patients suffering from unilateral alveolar cleft defects. These authors stated that their treatment had reasonable potential as a therapeutic option for alveolar bone cleft defects, but that further evaluation of the long-term effects was needed [39]. Focused on another application, Zhang and co-workers pre-seeded BM-MSCs on collagen sponges and applied cyclic mechanical stretch. Together with stimulation by TGF-β1, this mechanical stimulation synergistically promoted the differentiation of the cells into tenocytes and enhanced tendon regeneration when implanted into a rat Achilles tendon in an in situ repair model [40].
Collagen type I sponge-like scaffolds are also being tested for the delivery of the secreted products (secretome) produced by undifferentiated cells in culture. In one study, cell-derived nanovesicles were obtained by extruding umbilical cord-derived MSCs (ucMSCs) through nanoporous membranes, following a previously-described protocol [50]. These nanovesicles have certain similarities to exosomes but can be produced faster and in larger quantities. The authors loaded these nanovesicles together with BMP-2 on collagen sponges and implanted these in athymic nude mice calvarial defects. Their results showed that these nanovesicles can increase BMP-2-induced osteogenesis as they reported higher bone volumes, and more bone trabeculae and vessel-like structures than in the control implants [41].
Although it is well known that scaffolds designed for BTE should have an interconnected porosity and an appropriate pore size to allow osteoprogenitor cells to colonize the inner regions of the scaffolds and the growth of new blood vessels, it has recently been described that the orientation of the pores can also influence the osteogenic process [51]. With this in mind, we have recently produced and evaluated polymeric collagen type I sponge-like scaffolds with uni- or multidirectional pore orientations. The sponges with unidirectional, parallel pores had higher tensile strength, Young’s modulus and swelling capacity than their multidirectional counterparts. Furthermore, ectopic bone formation was significantly increased in the unidirectional sponges when loaded with BMP-2 and intramuscularly implanted in rats, with a parallel arrangement of the new-formed bone trabeculae, resembling the disposition found in cortical bone (yet unpublished) [52].
3. Crosslinking of Collagen Type I-Based Biomaterials
Collagen type I is extremely abundant in nature, easy to isolate and highly soluble in acidic solutions. This has allowed for the fabrication of a great variety of different collagen scaffolds for tissue engineering, such as collagen sponges, tubes, sheets, hydrogels, injectable solutions, nanoparticles, pellets, or tablets. The manufacturing and details of their fabrication processes have been reviewed previously [53][54][55]. In the specific field of BTE, one of the characteristics an ideal biomaterial must possess is a degradation rate in accordance with the time needed for the patient’s body to form biomechanically stable new bone.
Osteogenesis is a complex, multifactorial process that requires a significant amount of time. After a first inflammatory stage, characterized by the expression of pro-inflammatory cytokines and in which macrophages, lymphocytes, and other cells from the immune system invade the implant site, progenitor cells will migrate and differentiate within the implant to start the first stages of the osteogenic phases. The implanted biomaterial must remain undegraded, at least during these initial phases, to act as a support for the cells to proliferate and/or differentiate on. However, polymeric collagen type I is naturally very susceptible to biodegradation as it is a target for multiple collagenase enzymes that are being expressed by a variety of cells. Therefore, one very important step during the manufacturing of collagen-based scaffolds is crosslinking to reduce the natural biodegradation process and to increase the mechanical strength and stability by establishing intermolecular bonds [56]. Furthermore, crosslinking of collagen has shown to increase its capacity to support angiogenesis [57]. It is well known that osteogenesis is strictly dependent on successful vascularization of the implants and achieving this is one of the greatest challenges of modern BTE [58]. Although collagen type I itself already stimulates angiogenesis in vitro and in vivo [59], different strategies have been tested for increasing the vascularization of collagen-based biomaterials [60][61].
Crosslinking of collagen materials can be achieved using physical methods, such as dehydrothermal treatments [62][63] or ultraviolet light irradiation [64]. Since these methods are toxin-free, they could be considered very suitable for crosslinking collagen for biomedical use. However, physical crosslinking methods can only lead to a mild crosslinking between the collagen fibers, which is generally insufficient to prevent fast degradation in vivo. Hence, collagen scaffolds used for BTE are mostly crosslinked using chemical treatments, such as glutaraldehyde, isocyanates, or carbodiimides [65][66][67]. Although these methods are able to lower the degradation rate of collagen scaffolds enough to allow neo-osteogenesis within them, they are cytotoxic and non-reacted or side-produced residual molecules that remain in the scaffold might trigger undesired effects after implantation, such as inflammation or encapsulation [67][68]. The main methods for crosslinking collagen scaffolds have been recently analyzed by Adamiak & Sionkowska [56] and are summarized in
Table 2. Main methods for crosslinking collagen, according to [56].
| Type of Crosslinking | Main Characteristics | |
|---|---|---|
| Chemical | Glutaraldehyde (GA) | Low cost. High reactivity. High water solubility. Cytotoxic. |
| Genipin | Less toxic than other chemical crosslinkers. Might promote osteoblastic differentiation. Not suitable for gelatin crosslinking. | |
| 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS). | Zero-length crosslinker. Acts mainly as an intra-fibrillar crosslinker. Low cytotoxicity. | |
| Dialdehyde starch | Can be used for intra- and intermolecular crosslinking. Low cytotoxicity. Biodegradable. Has antiviral activity. | |
| Chitosan | Biodegradable. Non-toxic. Has antibacterial and antifungal activity. Poor water solubility. Tends to form polydisperse solutions. | |
| Physical | Dehydrothermal (DHT) treatment | Non-toxic. Provides sterilization. May cause collagen denaturation. |
| UV light | Faster than DHT. Non-toxic. Provides sterilization. May cause collagen denaturation. | |
| Enzymatic | Microbial transglutaminase | Similar to natural crosslinking. More expensive. |
To avoid some of the problems related to the use of high concentrations of chemical crosslinkers, we have recently fabricated a collagen type I biomaterial for BTE in the form of sponge-like scaffolds, which were crosslinked with a combination of dehydrothermal and chemical glutaraldehyde crosslinking methods [69]. This allowed us to use a concentration of glutaraldehyde significantly lower than the ones generally used. In addition, these sponges were produced from purified, bovine atelocollagen. Frequently, collagen-based biomaterials used for BTE are fabricated using polymeric collagen extracted from animal tissues and the general tendency is to keep these molecules as close to their native conformation as possible. However, these collagen fibrils keep N- and C-terminal telopeptides, which have been shown to be responsible for most of the immune reactions reported against collagen [70]. Hence, the use of purified atelocollagen, together with the combined physical and chemical crosslinking, might help overcome some of the drawbacks of many collagen sponges used currently in BTE approaches.

Figure 3.
A
B
p
C
a
b
s
t
arrows: osteocytes. Modified with permission from [69].
