Liquid crystal elastomer (LCE) describes a class of materials that combine the elastic entropy behaviour associated with conventional elastomers with the stimuli responsive properties of anisotropic liquid crystals. LCEs consequently exhibit attributes of both elastomers and liquid crystals, but additionally have unique properties not found in either. Recent developments in LCE synthesis, as well as the understanding of the behaviour of liquid crystal elastomers—namely their mechanical, optical and responsive properties—is of significant relevance to biology and biomedicine. LCEs are abundant in nature, highlighting the potential use of LCEs in biomimetics. Their exceptional tensile properties and biocompatibility have led to research exploring their applications in artificial tissue, biological sensors and cell scaffolds by exploiting their actuation and shock absorption properties. There has also been significant recent interest in using LCEs as a model for morphogenesis.
- liquid crystal elastomers
- biological materials
- auxetics
- biomimetics
- actuators
1. Introduction
Liquid crystal elastomers (LCEs) are a novel class of materials that combine the properties of liquid crystals (which exhibit orientational order) with the elastic properties of conventional elastomers [1]. As a result, they display unique and interesting responses to a variety of external stimuli, as well as intriguing responses to mechanical deformation, such as semi-soft elasticity, auxeticity and actuation properties. The exceptional potential of these elastomers is constantly being expanded on as more advanced synthesis and characterisation techniques are being developed. An interesting example is recent tensile rig developments which have allowed the concurrent analysis of the tensile behaviour and the liquid crystal texture. The resulting insight into the strain-dependent liquid crystal director reorientation, the mechanical properties and the birefringence (and hence the liquid crystal order parameter), led to a re-evaluation of deformation modes that occur in different LCE systems [2]. The exceptional properties of LCEs, such as stimuli responsiveness and actuation, have shown them to be versatile materials for use in a range of applications in the fields of biology and medicine, from artificial muscles to the control of cell maturation during cell culture [3]. This review article will cover some of the key discoveries and advances of LCEs in the field of biology.
2. Liquid Crystal Elastomers for Biomedical Applications
The Poisson’s ratio (ν) of a material is defined as the negative ratio of the transverse strain to the axial strain in the direction of loading. For many materials, this value is positive and reflects a need to conserve volume. Materials with a negative Poisson’s ratio display the unexpected property of lateral expansion when stretched, rather like a Hoberman® sphere, with an equal and opposing densification when compressed [4][5]. FigureFigure 6 1 presents a schematic of the behaviour of auxetic and non-auxetic materials.
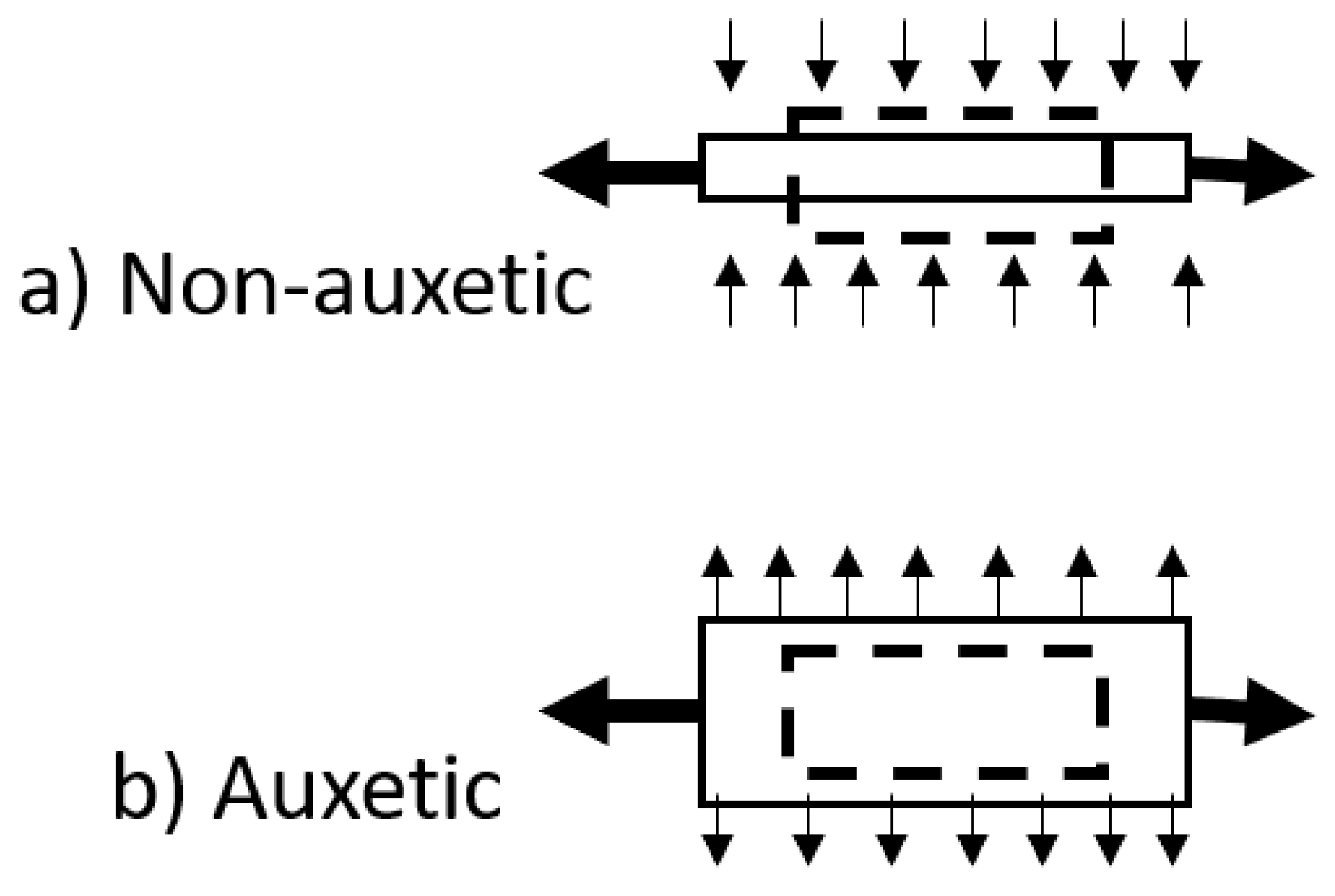
Figure 16. Schematic diagram of positive and negative Poisson’s ratio deformation. (a) Non-auxetic behaviour in which an initially undeformed material (dashed outline) undergoes longitudinal extension and lateral contraction (solid line) for a tensile load applied in the longitudinal (x) direction. (b) Auxetic behaviour in which an initially undeformed material (dashed outline) undergoes longitudinal and lateral extension (solid line) for a tensile load applied in the longitudinal (x) direction.
Materials with a negative Poisson’s ratio are referred to as auxetic materials. The vast majority of auxetic materials are cellular auxetics. Cellular auxetics are porous materials, where a volume increase results from the reorganisation of the internal cellular structure and a reduction in density. The auxeticity in molecular auxetics, however, arises from changes in their microstructure, perhaps resulting from molecular segments rotating upon deformation [6]. In molecular auxetics, only one axis shows a negative Poisson’s ratio, and this is coupled to a large contraction in another axis to maintain constant volume and the original sample density.
Until recently, molecular auxetics were only found in nature, for example, in 69% of cubic metals, α-cristobalite, and numerous examples of the zeolite class of materials [7][8][9]. Cellular auxetics are abundant in nature; for example, this behaviour has been reported in cow teat skin [10], cat skin [11], cancellous bone [12], tendons [13] and membranes found in the cytoskeleton of red blood cells [13][14]. Unlike molecular auxetics, many examples of synthetic cellular auxetics exist, such as the Hoberman® sphere [4], ultra-high molecular weight polyethylene (UHMWPE) [15] and paper [16].
Recently, studies have identified the benefits of using auxetic materials in skin healing [17]. Skin healing is facilitated by the migration of cells to the wound site. Small simple wounds, such as small finger-pricks, rarely need assistance in healing. However, when complex wounds occur, such as burns, or when more widespread tissue regeneration is required, assistance can promote faster healing and minimise scarring. Tissue regeneration can be facilitated if a suitable scaffold is provided, the desired cells replicate more easily and grow along the predefined structure. Polylactic acid-based fibres which can be produced by electrospinning are cellular auxetics [17][18]. The auxeticity of these scaffolds are particularly attractive because of their ability to apply an enhanced negative pressure on the wound site.
Auxetic skin sensors have also recently been developed [19]. The auxetic nature of these skin sensors is particularly appealing because they display excellent mechanical compliance to dynamic body motions. Human skin displays a negative Poisson’s ratio in some regions—expanding biaxially during bending, exhalation and muscle tension. Often, polymer films are unable to maintain contact with the skin under large body motions due to their positive Poisson’s ratio. The usage of auxetic sensors can therefore maintain conformational contact [19].
Recently, a side-chain LCE was the first ever synthetic material to display molecular auxetic behaviour [2]. This polyacrylate-based LCE showed that volume was conserved when strained, confirming that the material was a molecular auxetic. The Poisson’s ratio displayed by the material was up to −0.8. The auxetic behaviour was not observed at low strains, but at higher strains (>80%). Once the strains necessary to display auxetic behaviour of the LCE were reached, a coincident negative order parameter was also observed. It is believed that the auxetic response is related to the inherent Mechanical Fréedericksz transition displayed by some LCEs [2]. FigureFigure 7 2 shows the change in the thickness (strain in the z-direction, εz) of the polyacrylate based auxetic LCE in response to strain in the x-direction, εx. Shown also is the change in birefringence as a function of strain, displaying a decrease in retardance as the strain is increased, reaching a minimum at εx = 1.14, where what is believed to be a negative order parameter is observed. A further increase in εx results in the retardance increasing again. The relationship between the liquid crystal order and the emergence of auxetic behaviour of the LCE is still being explored. The reason as to why some LCEs display the Mechanical Fréedericksz transition whereas others do not is also currently unclear.
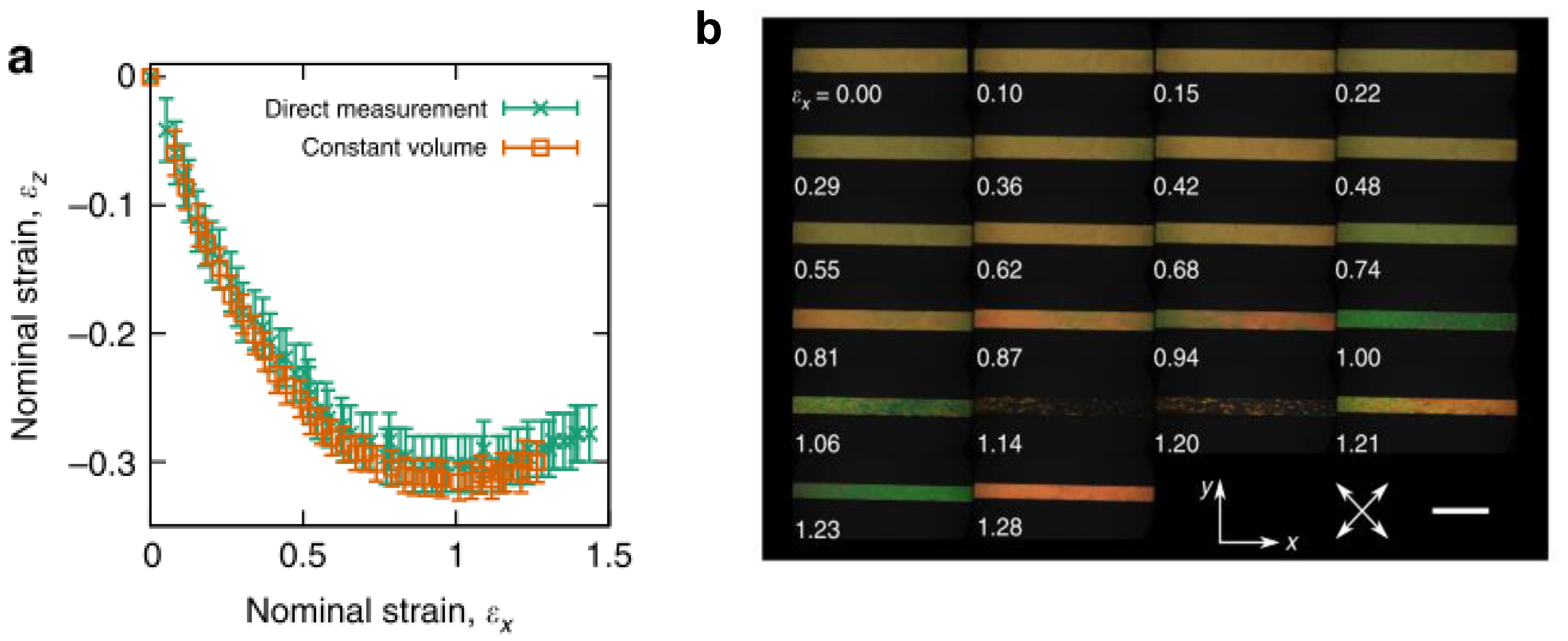
Figure 27. (a) z-axis strain, εz in response to imposed x-axis strain, εx, measured via direct observation of the polyacrylate-based auxetic LCE in the xz plane (green crosses) and through the application of the constant volume condition to strain measurements of the xy plane (orange boxes). Errors are measurement errors (n = 1). (b) Negative order from polarising microscopy. Polarising microscopy textures at different strains. The birefringence colours indicate the retardance initially decreases, becoming zero at εx = 1.14, before increasing again. Scale bar, 5 mm. (c) shows the constituents used to synthesise the auxetic LCEs reproduced with permission from Nature from Mistry et al. [2].
These auxetic LCEs show exciting potential for biomimetic applications. As previously discussed, the auxeticity of materials used in skin sensors and skin healing scaffolds significantly enhances their performance compared to the non-auxetic counterparts. Molecular auxetics could be used in composites which mimic the stress-strain and auxetic behaviour of human skin [19]. Conventional auxetics have an “open” microstructure, which confers reduced mechanical properties [9]. In many cases, these “open” microstructures display a tensile strength that is too weak for practical applications due to their microscale porosity; however, these limitations could be circumvented by molecular auxetics due to their “closed” microstructure.
Another potential application is the use of LCEs as artificial blood vessels; the auxeticity would allow the vessels to withstand the high pressure of blood through the vessels, which are less prone to rupture as a result of thinning [20].
Furthermore, a range of tissues display auxetic behaviour [13][14]. The discovery of the inherent auxeticity of these tissues has a range of implications for tissue engineers in mimicking the properties of these auxetic tissues. The mechanical characteristics of engineered tissue ideally should match or enhance the mechanical properties of healthy, normal host tissues, permitting full functionality, enabling it to fulfil its role in vivo. Cells exist in their natural in vivo environment embedded within an extracellular matrix, which is the natural scaffold of the body produced by the cells within tissues. Therefore, if the target tissue is auxetic, an auxetic scaffold would most closely match the properties of this tissue. The matching of this characteristic would be beneficial in recreating the loading environment that cells would naturally experience. As will be discussed in further detail later in this review, LCEs have already been considered and have shown to be suitable for use as tissue replacements, as their actuation properties make them ideal candidates for use as artificial muscles. Exploiting the auxeticity of some LCEs is, therefore, promising for potential applications in tissue engineering.
This discovery of a molecular auxetic has overcome a long-standing limitation in the auxetics industry and there is, therefore, a lot of potential for these materials not just in biomedicine, but in the wider materials world [21][22]. The main challenge lies within understanding and, if necessary, making adaptations to ensure the biocompatibility of the materials.
3. Conclusions
In 1975, six years before the synthesis of the first ever LCEs, the significance of LCEs was highlighted by de Gennes, who presented a theoretical framework of artificial muscle-base on the contraction of LCEs at the nematic-isotropic transition. Since then, incredible advances have been made in the field of LCEs to make this a reality. Studies have explored the possibility of stimuli-responsive LCEs, and in more recent years, in vitro and even in vivo testing has begun to take place, providing proofs of concept in the usage of LCEs as actual artificial muscles. Although they have not yet been used in a medical environment, the impressive developments thus far imply that this could be a possibility in the near future. The significance of LCEs in biology is not only limited to artificial muscles; a more recent discovery of a molecular auxetic LCE has also opened up the possibility for the use of LCEs as general artificial tissues. In addition to this, LCEs have, unsurprisingly, been found to exist in nature, providing exceptional physical properties. This highlights the need for further research on understanding the structure and behaviour of these natural LCEs, the findings of which may allow for the synthesis of materials with exceptional mechanical and tensile properties, potentially on a commercial level.
References
- Warner, M.; Terentjev, E. Liquid Crystal Elastomers; Clarendon Press: Oxford, UK, 2007; Volume 1, ISBN 9788578110796.
- Mistry, D.; Connell, S.D.; Mickthwaite, S.L.; Morgan, P.B.; Clamp, J.H.; Gleeson, H.F. Coincident molecular auxeticity and negative order parameter in a liquid crystal elastomer. Nat. Commun. 2018, 9, 1–9.
- Prévôt, M.E.; Ustunel, S.; Hegmann, E. Liquid crystal elastomers-A path to biocompatible and biodegradable 3D-LCE scaffolds for tissue regeneration. Materials 2018, 11, 377.
- Huang, C.; Chen, L. Negative Poisson’s Ratio in Modern Functional Materials. Adv. Mater. 2016, 28, 8079–8096.
- Li, Y.; Chen, Y.; Li, T.; Cao, S.; Wang, L. Hoberman-sphere-inspired lattice metamaterials with tunable negative thermal expansion. Compos. Struct. 2018, 189, 586–597.
- Novak, N.; Vesenjak, M.; Ren, Z. Auxetic Cellular Materials—A Review. Stroj. Vestn. J. Mech. Eng. 2016, 62, 485–493.
- Baughman, R.H.; Shacklette, J.M.; Zakhidov, A.A.; Stafstro, S. Negative Poisson′s ratios as a common feature of cubic metals. Nature 1998, 392, 362–365.
- Grima, J.N.; Gatt, R.; Zammit, V.; Williams, J.J.; Evans, K.E.; Alderson, A.; Walton, R.I. Natrolite: A zeolite with negative Poisson′s ratios. J. Appl. Phys. 2007, 101, 101–104.
- Yeganeh-Haeri, A.; Weidner, D.J.; Parise, J.B. Elasticity of α-cristobalite: A silicon dioxide with a negative poisson′s ratio. Science 1992, 257, 650–652.
- Lees, C.; Vincent, J.F.; Hillerton, J.E. Poisson′s ratio in skin. Biomed. Mater. Eng. 1991, 1, 19–23.
- Veronda, D.R.; Westmann, R.A. Mechanical characterization of skin-Finite deformations. J. Biomech. 1970, 3, 111–124.
- Williams, J.L.; Lewis, J.L. Properties and an anisotropic model of cancellous bone from the proximal tibial epiphysis. J. Biomech. Eng. 1982, 104, 50–56.
- Gatt, R.; Vella Wood, M.; Gatt, A.; Zarb, F.; Formosa, C.; Azzopardi, K.M.; Casha, A.; Agius, T.P.; Schembri-Wismayer, P.; Attard, L.; et al. Negative Poisson’s ratios in tendons: An unexpected mechanical response. Acta Biomater. 2015, 24, 201–208.
- Mardling, P.; Alderson, A.; Jordan-Mahy, N.; le Maitre, C.L. The use of auxetic materials in tissue engineering. Biomater. Sci. 2020, 8, 2074–2083.
- Alderson, K.L.; Pickles, A.P.; Neale, P.J.; Evans, K.E. Auxetic polyethylene: The effect of a negative poisson′s ratio on hardness. Acta Metall. Mater. 1994, 42, 2261–2266.
- Verma, P.; Shofner, M.L.; Griffin, A.C. Deconstructing the auxetic behavior of paper. Phys. Status Solidi Basic Res. 2014, 251, 289–296.
- Domaschke, S.; Morel, A.; Fortunato, G.; Ehret, A.E. Random auxetics from buckling fibre networks. Nat. Commun. 2019, 10, 1–8.
- Higuchi, J.; Fortunato, G.; Wozniak, B.; Chodara, A.; Lojkowski, W. Biological Barrier Membrane. WO 2020/085927 A1, 30 April 2020.
- Kim, H.W.; Kim, T.Y.; Park, H.K.; You, I.; Kwak, J.; Kim, J.C.; Hwang, H.; Kim, H.S.; Jeong, U. Hygroscopic Auxetic On-Skin Sensors for Easy-to-Handle Repeated Daily Use. ACS Appl. Mater. Interfaces 2018, 10, 40141–40148.
- Umakiran, T.; Kumar, A. Auxetic Textile and its Applications. Man Made Text. India 2009, 52, 265.
- Evans, K.E.; Alderson, K.L. Auxetic materials: The positive side of being negative. Eng. Sci. Educ. J. 2000, 9, 148–154.
- Mcmullan, P.J.; Kumar, S.; Griffin, A.C. NTC Project: M04-GT21 Textile Fibres Engineered From Molecular Auxetic Polymers National Textile Center Annual Report; National Textile Center: Philadelphia, PA, USA, 2005; pp. 1–10.
