Chitosan is a random copolymer comprising d-glucosamine (the deacetylated ones) and N-acetyl-d-glucosamine units.
- chitosan
- chitosan oligosaccharides
- gut microbiot
1. Introduction
Chitosan oligosaccharides (COS) are a mixture of oligomers of chitosan with an average molecular weight (MW) < 5000 Da; their structural units are also GlcNAc and 2-amino-2-deoxy-β-
d
-glucopyranose (GlcN)
. Chitosan oligosaccharide is readily soluble due to its short chain length, unlike chitin
[3]
. Chitosan and chitosan oligosaccharides have been widely investigated in recent reviews that have shown several crucial bioeffects, e.g., antibacterial
, anti-inflammatory
, and anti-diabetic
activities. These biological reactions are brought about by the physicochemical properties such as favorable water solubility and cationic nature
[2]
.
Chitosan and its derivatives serve as prebiotics, nondigestible carbohydrates which selectively stimulate the growth of beneficial microbes
[9]
. Polysaccharides cannot be digested by digestive enzymes in the gastrointestinal tract but can be fermented by gut microbiota. The gut microbiome encodes the genome for hydrolyzing nondigestible carbohydrate. Additionally, different microbes containing different enzymes are capable of fermenting corresponding carbohydrates, which is a case of a symbiotic relationship between the host and the intestinal flora
[10]
. Furthermore, the degree of polymerization influences the location of fermentation in the gastrointestinal tract. Namely, soluble oligosaccharides tend to be fermented in proximal segments of the gastrointestinal tract, while less soluble polysaccharides tend to be fermented in the distal colon
[10]
. In general, polysaccharides and their derivatives have been advantageous to the gut microbiota.
Recent studies are focused on the effects of prebiotics on the composition of the gut microbiota. Prebiotics selectively promote the growth of beneficial enteric bacteria (e.g.,
Lactobacillus
,
Bifidobacterium
, and
Faecalibacterium
[11]
) and inhibit the occupation of harmful bacteria such as the genus
Desulfovibrio
[12]
(phylum
Proteobacteria
). Polysaccharides with increasing populations of beneficial intestinal flora have been described in several recent reviews
[10]
. Concretely, oligosaccharides such as oligofructose and xylo-oligosaccharides could support the growth of beneficial bacteria, e.g.,
Lactobacillus
,
Bifidobactetrium
,
Bacteroides
[13]
. Furthermore, previous studies showed that chitosan and its derivatives could increase the population of probiotic genera
Bifidobacterium
[12]
,
Lactobacilli
[14]
, genus
Akkermansia
[15]
, and
Parabacteroides
[12]
.
There is a consensus that gut dysbiosis damages the intestinal barrier. For instance, mucin glycoprotein, especially mucin 2 (MUC2), produced by goblet cells to the lumen of the intestine, is dominant in the composition of intestinal mucus. It also constitutes the inner layer of intestinal mucus that contributes to the intact intestinal barrier and intestinal homeostasis
[16]
. A previous study has shown that mucins in the mucosa of germ-free mice can be attenuated due to gut dysbiosis, thus leading to the inflammation of the intestinal mucosa, increased mucosal permeability, and severe immune responses in lymphoid tissues
[16]
. Mucins compete with microbes (pathogens and commensals) for binding sites to the surface of the underlying epithelial lining, which prevents bacterial translocation across the intestinal barrier
[17]
. Mucins are exploited as a carbon source by the gut microbiota predominantly in the distal colon, hence promoting the growth of bacteria in the outer loose mucus and thickening the outer layer of intestinal mucus
. Therefore, mucin-degrading bacteria can decompose mucins into sugars and produce short-chain fatty acids (SCFAs), which offer energy to intestinal epithelial cells
[19]
, thus contributing to the intact epithelial lining
[20]
. Recently, a mucin-degrading bacterium,
Akkermansia muciniphila
, has attracted considerable attention from many researchers due to its ability to lower blood lipids and blood glucose
. Recent evidence has suggested that the abundance of
Akkermansia muciniphila
in mouse models is inversely associated with obesity
[21]
, insulin resistance
[22]
, hepatic steatosis
[23]
, and atherosclerosis
[24]
, among others. Apart from the reduction of
Akkermansia muciniphila
, the dysregulation of the intestinal flora is closely corelated with diet-associated abnormalities
[25]
. The clustering of these pathologic conditions is categorized as metabolic syndrome
[25]
, also known as syndrome X, a term depicting features that increase the risks of cardiovascular disease
[25]
. The diagnosis of metabolic syndrome requires at least three of the following clinical findings: abdominal obesity, increased fasting glucose, hypertension, high-density lipoproteins (HDLs), and hypertriglyceridemia
[26]
. Existing therapies for metabolic syndrome are mainly treatments for hyperlipidemia, hyperglycemia, hypertension, as well as dietary management and regular exercise
[27]
. There are three phases in a pathophysiological overview for metabolic syndrome: risk factors (overnutrition, physical inactivity, smoking, age, ethnicity, etc.); inflammation-induced insulin resistance, oxidative stress, mitochondrial dysfunction, etc.; type 2 diabetes, hypertension, dyslipidemia, non-alcoholic fatty liver disease (NAFLD)
[28]
, etc. The pathophysiology of metabolic syndrome is associated with chronic inflammation in adipose tissue, vascular endothelium, etc., hence leading to cardiovascular diseases (e.g., atherosclerosis)
[27]
.
In previous studies, in hosts with diabetes
[29]
, hyperlipidemia, hepatic steatosis
[30]
, atherosclerosis
[24]
, and other clinical findings that are categorized under metabolic syndrome, it was observed that beneficial bacteria declined, intestinal mucus was attenuated, and harmful proinflammatory microbes increased in the intestinal mucosa. The prevalence of metabolic syndrome has displayed a worldwide growing trend: around one third of US adults have metabolic syndrome and the percentage of overweight and obese people in China showed a growth from 14.6% to 21.8% within 10 years. It is estimated that around one quarter of the world’s population (more than one billion people) suffers from metabolic syndrome
[31]
. Thus, it is a question of great interest to develop new therapies for metabolic syndrome
[32]
.
Traditionally, type 2 diabetic patients take anti-diabetic drugs, especially metformin. Recent research supported the notion that metformin acted on the regulation of gut microbiota patterns by promoting the abundance of mucin-degrading
Akkermansia muciniphila
as well as various SCFA-producing microbes
[33]
. Chitosan and its derivatives play the same role in the intestinal microbiota. Recent studies reported several adverse reactions of metformin, such as metabolic acidosis and hyperlactatemia, because of obstructed elimination from the body, which would exert an additive effect to increase circulating metformin body levels
[34]
. There have been no chitosan-induced adverse reactions reported to date. Hence, chitosan and its derivatives have an underlying capacity for the attenuation of elevated fasting glucose.
Likewise, statins play an important role in lipid-lowering through alterations of the gut microbiota
[35]
. Recent studies illustrated that atorvastatin and rosuvastatin, mostly prescribed statins, induced significant changes in the increased abundance of genera
Bacteroides
,
Butyricimonas
, and
Mucispirillum
which are associated with decreased cholesterols and triglycerides
[35]
. Similarly, an increasing trend of the population of
Akkermansia muciniphila
was also found in atorvastatin-treated hypertension patients
[36]
. However, atorvastatin, the most prescribed medication, could induce muscle-related adverse reactions
[37]
, myopathy, and even rhabdomyolysis. Additionally, the co-administration of statins and fibrates causes over 10-fold increased incidence of rhabdomyolysis
[38]
, whereas the administration of chitosan and its derivatives can lower lipids in plasma through modification of the gut microbiota without noticeable adverse reactions
[39]
. Vitamin E, an antioxidant for the treatment for non-alcoholic fatty liver disease (NAFLD), was reported to influence gut microbiota. At phylum level, the population of
Proteobacteria
, comprising various pathogens such as
Escherichia coli
and
Salmonella
, was positively associated with increased consumption of vitamin E. The decreased abundance of
Verrucomicrobia
, including mucin-degrading
Akkermansia Muciniphilia
, was observed in vitamin-E-treated groups in mouse models. Recently, a quaternized chitosan derivative was found to have potent antioxidant effects
[40]
. Therefore, chitosan and its derivatives can act as an alternative in the treatment for NAFLD. Overall, chitosan and its derivatives may be applied as a potential adjuvant for metabolic syndrome treatment due to their innocuous properties.
2. Biological Effects of Chitosan and Its Derivatives
Chitosan and chitosan oligosaccharides can be utilized to alleviate metabolic syndrome through alterations of the gut microbiota, reducing inflammation in the intestinal mucosa, and decreasing the risks of cardiovascular disease
.
The bioeffects of chitosan and its derivatives come from the positively charged NH
3+
group. The positive charge on the molecules neutralizes the negative charge on the proteins, lipids, and other substances with negatively charged radicals, which occupy critical positions in significant metabolism and other vital reactions
[43]
. Phospholipids on the surface of the membrane are negatively charged; hence, chitosan is capable of interacting with cells in multiple intercellular bioreactions
[43]
. At the molecular level, phosphate groups in deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) have a negative charge
[44]
, and proteins in extracellular fluid (pH > 7) are negatively charged due to carboxyl groups
[45]
. Therefore, chitosan can react to negatively charged cells and macromolecules. Molecular weight (MW) influences the efficiency of regulating lipid metabolism in chitosan-treated groups with high-fat diet (HFD)
[46]
. Previous studies showed that high-MW chitosan had a higher efficiency than low-MW chitosan in the inhibition of lipid absorption and enhancement of fatty acid oxidation in the liver
[46]
. High deacetylation reduces the chitosan–triglyceride interaction due to reduced intermolecular hydrophobic forces between
N
-acetyl-
d
-glucosamine units (acetylated groups) and triglycerides
[47]
. Therefore, an increased degree of deacetylation implies decreased oil-binding abilities.
2.1. Lipid-Lowering Effects of Chitosan and Its Derivatives
Hyperlipidemia and hyperglycemia, characterized by high serum cholesterol, triglycerides, or low-density lipoprotein (LDL), is an important risk factor associated with cardiovascular diseases [48][49].
Traditional lipid-lowering medication such as statins might mitigate hyperglycemia and hyperlipidemia through alterations of the gut microbiota [35]. Recent studies have shown that statins increase the abundance of beneficial microbes which are associated with decreased serum cholesterols and triglycerides [35]. Several muscle-related adverse events occurred after the administration of statins [50], whereas the administration of chitosan and its derivatives can lower serum lipid levels through the modification of the gut microbiota without noticeable adverse reactions [39]. Recently, the literature on chitosan-induced lipid-lowering effects has been reviewed.
The administration of chitosan reduced total cholesterol (TC) by 8%, low-density lipoprotein cholesterol (LDL-C) by 2%, and triglyceride (TG) by 19%, and it increased high-density lipoprotein cholesterol (HDL-C) by 14% [51]. Furthermore, chitosan has a positive influence on low-density lipoprotein (LDL) subtypes by increasing low-density lipoprotein-2 particles and decreasing atherogenic low-density lipoprotein. The low-density lipoprotein-2 particle is categorized as the large low-density lipoprotein, and the large size of low-density lipoprotein makes it less likely to be taken up by the arterial tissue than its smaller counterparts, suggesting less trans-endothelial transportation—namely, less atherogenic properties [51]. Chitosan can also inhibit the differentiation and the development of adipose cells by the gathering of triglycerides and expression of adipogenic epitopes. Furthermore, because cholesterol is a synthetic raw material for bile acids, chitosan can eliminate excess cholesterol and bile acids from the body by transferring them to the liver for excretion [52].
AMP-activated protein kinase (AMPK) plays a critical role in cellular metabolism in target tissues such as the liver, adipose tissue, and skeletal muscles [53], and it inhibits the deposition of fat by suppressing peroxisome proliferator-activated receptor-γ (PPAR-γ) and sterol regulatory element-binding proteins (SREBPs) [54]. In HFD hosts, Figure 2 illustrates that chitosan can activate AMPK and inhibit SREBPs and PPAR-γ [46]. Phosphorylation of AMPK induces the downregulation of adipogenic transcription factors (e.g., SREBPs and PPAR-γ), which lessens lipid synthesis and promotes lipolysis. PPAR-γ has promoted adipogenesis and triglyceride storage in adipocytes, while SREBPs can increase lipogenesis-related genetic expression such as fatty-acid-transport proteins, fatty-acid-binding proteins, fatty acid synthetase FAS, acetyl-CoA carboxylase, and HMG-CoA reductase [55][56].
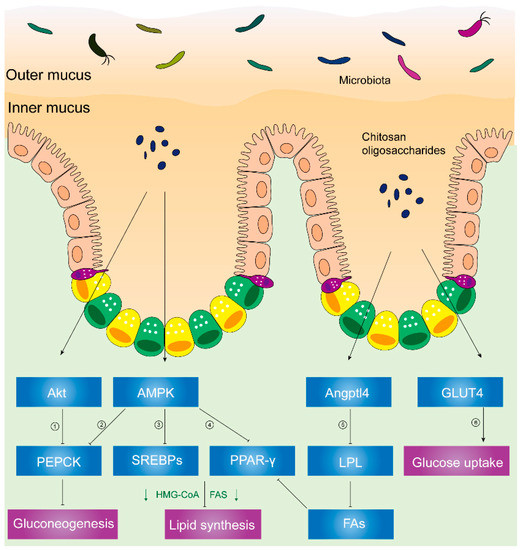
Figure 2. Chitosan and its derivatives mediate lipid and glucose metabolisms. ① Phosphorylated Akt can activate PEPCK, a rate-limiting enzyme in gluconeogenesis, and the conversion of noncarbohydrates to glucose. ② Activated AMPK can phosphorylate PEPCK. ③ Phosphorylated AMPK can inhibit expression of SREBPs, leading to inhibited HMG-CoA, FAS, etc. HMG-CoA is responsible for synthesis of cholesterol. FAS acts on synthesizing fatty acids. ④ Activated AMPK inhibits PPAR-γ, causing suppressed HMG-CoA, FAS, etc. ⑤ Upregulated Angptl4 inhibits activity of LPL, leading to less fatty acids released from VLDL and chylomicrons. Reduced fatty acids inhibit PPAR-γ, suppressing HMG-CoA, FAS, etc. ⑥ Translocation of GLUT4 from cytoplasm to skeletal muscle cell membranes can increase glucose uptake and decrease plasma glucose levels. Abbreviations: PEPCK, phosphoenolpyruvate carboxykinase; AMPK, AMP-activated kinase; SREBPs, sterol regulatory element-binding proteins; HMG-CoA, hydroxy methylglutaryl coenzyme A reductase; FAS, fatty acid synthetase; PPAR-γ, peroxisome proliferator-activated receptor-γ; Angptl4, angiopoietin-like 4; LPL, lipoprotein lipase; FAs, fatty acids; GLUT4, glucose transporter-4.
Furthermore, Figure 2 illustrates decreased activity of lipoprotein lipase (LPL), reduced microsomal triglyceride transfer protein (MTTP) expression, and increased angiopoietin-like 4 (Angptl4) expression [54] in chitosan-treated mice.
Angptl4, a glycosylated and secreted protein, is also known to be an endogenous inhibitor of LPL and thereby alters plasma TG values. Angptl4 can be divided into several distinct regions: an N-terminal domain which contains two coiled-coil domains, a linker region, and a C-terminal fibrinogen-like domain [57][58]. Angptl4 protein is proteolytically cleaved by proprotein convertases (PCs) into different forms due to different patterns of the C-terminal and N-terminal domains. Angptl4 is distributed separately in various organisms, suggesting that the different patterns of Angptl4 may be associated with distinct species’ biological functions [58]. Additionally, full-length Angptl4 (flAngptl4, 50 kDa) and the yielded fragments, an N-terminal (nAngptl4) and a C-terminal (cAngptl4) fragment, indicate different functions in metabolism [59]. It was reported that the N-terminal helical region of Angptl4 is necessary to inhibit LPL [60]. In agreement with this notion, recent evidence has suggested that nAngptl4 and flAngptl4 inhibit LPL activities, although there is an absence of evidence showing the biological effects of cAngptl4 on lipid metabolism [59].
LPL, an enzyme attached to the luminal surface of endothelial cells in capillaries, is capable of decomposing TG and releasing free fatty acids, chylomicrons, and the very low-density lipoprotein (VLDL) particles [61]. Thereafter, increased free fatty acids can serve as PPAR-γ agonists and Angptl4 activators [62][63]. MTTP, an endoplasmic reticulum (ER)-resident protein, can transport lipids (e.g., TG, cholesteryl esters, free cholesterol, phospholipids, ceramides, and sphingomyelin), hence facilitating the optimal formation of lipoproteins [64]. Previous studies showed that the biological functions of absorbing and assembling chylomicrons into enterocytes are impaired in MTTP-IKO mice [65].
As shown in Figure 2, chitosan upregulated the Angptl4 protein expression, inhibiting LPL activity. Hence, reduced free fatty acids which come from VLDL and chylomicrons can inhibit the phosphorylation of PPAR-γ. Thereafter, chitosan promoted fat liberation and decreased fat storage. Consequently, plasma lipids and liver fat accumulation decreased due to the administration of chitosan [54].
2.2. Anti-Diabetic Effects of Chitosan and Its Derivatives
Insulin resistance, recognized as metabolic syndrome, is a contributing factor to the etiology of type 2 diabetes mellitus (T2DM) and is associated with a wide range of other pathogenic conditions including hypertension, hyperlipidemia, and others [66].
Type 2 diabetes mellitus, previously termed “noninsulin-dependent diabetes”, is a metabolic disease characterized by hyperglycemia [67]. Previous studies showed that the etiology of type 2 diabetes mellitus is related to β-cell dysfunction, chronic low-degree inflammation, and mitochondrial oxidative stress Patients with type 2 diabetes mellitus may initially not need insulin treatment to survive, even throughout their entire lifetime [67].
Traditionally, type 2 diabetic patients take anti-diabetic drugs, especially metformin. Recent research supported the notion that metformin modifies gut microbiota patterns by increasing the population of mucin-degrading Akkermansia muciniphila as well as various SCFA-producing microbes [33]. Chitosan and its derivatives impose similar effects on the intestinal microbiota without adverse reactions, whereas metformin was found to induce metabolic acidosis and hyperlactatemia because the obstructed elimination can exert an accumulative effect on plasma metformin levels [34]. Hence, chitosan and its derivatives have an underlying capacity to decrease fasting glucose.
In a recent randomized, controlled, double-blind, crossover clinical trial, the administration of chitosan oligosaccharides could reduce postprandial blood glucose levels in the 2-h oral sucrose tolerance test (OSTT), suggesting the improvement of impaired glucose tolerance [68]. Similarly, chitosan has reduced gluconeogenesis-related signals (e.g., PEPCK (phosphoenolpyruvate carboxykinase), p38, and AMPK), increased muscle glucose uptake-related signals (e.g., Akt), and promoted glucose transporter-4 (GLUT4) translocation from cytoplasm to membranes in streptozotocin-induced diabetic rats. Akt, known as protein kinase B or PKB [69], plays a critical role in various cellular processes. Phosphorylated Akt promotes glucose uptake and inhibits gluconeogenesis in response to insulin secretion [69][70]. Glucose uptake is predominantly mediated by the translocation of GLUT4 from vesicular intracellular compartments to cell membranes in skeletal muscles [69]. Phosphorylated Akt or AMPK activates PEPCK, a rate-limiting enzyme in gluconeogenesis, and the conversion of noncarbohydrates to glucose [70][71].
Therefore, chitosan is capable of inhibiting gluconeogenesis by inhibiting PEPCK and triggering glucose uptake in skeletal muscles through the translocation of GLUT4 from the cytoplasm to the cell membrane in diabetic rat models, thus lowering blood glucose [72].
2.3. Anti-Inflammatory Effects of Chitosan and Its Derivatives
Exogenous substances and tissue damage can initiate inflammation, followed by the generation of proinflammatory cytokines, immune cell recruitment and activation, and free radical production [73].
Nonsteroid anti-inflammatory drugs (NSAIDs) which inhibit cyclooxygenase (COX) are widely used as analgesics and antiplatelet agents. It was reported that approximately 12.8% of US adults (29.4 million) used NSAIDs at least three times a week for at least 3 months [74]. Chronic inflammation plays a critical role in various biological reactions (e.g., insulin resistance) and tissues (e.g., adipose tissue), triggering metabolic syndrome. However, NSAIDs can damage the intestinal mucosa and even cause alimentary tract hemorrhage [75]. Consequently, significant alterations of the gut microbiota in NSAID-treated hosts were found in recent research. Multiple in vivo and in vitro studies demonstrated that chitosan oligosaccharides could inhibit inflammatory reactions in response to lipopolysaccharide (LPS) or other stimuli [76] via AMPK phosphorylation [74]. Additionally, COS can inhibit TNF-induced (tumor-necrosis-factor-induced) NF-κB (nuclear-factor-κB) signaling, COX-2 and iNOS (inducible nitric oxide synthase) [74]. COX-2, a prostaglandin (PG)–endoperoxide synthase 2 enzyme, contributes to the production of prostanoid-like prostaglandin E2 (PGE2), which triggers inflammation [77]. iNOS is an enzyme that is responsible for nitric oxide (NO) synthesis.
2.4. Application of Chitosan and Its Derivatives in Clinical Trials
Previous clinical trial results make chitosan and its derivatives more likely to be used as alternative applications in the treatment of metabolic syndrome. In a randomized, double-blinded, placebo-controlled, crossover clinical trial, chitosan could lower total cholesterol (TC) levels and low-density lipoprotein cholesterol (LDL-C) concentrations in plasma
[78]
. In a recent clinical trial, after the administration of chitosan oligosaccharide (GO2KA1, a code name of chitosan oligosaccharide), it showed a decreasing trend in body fat ratios and waist circumferences, serum glucose levels, HbA1c (Hemoglobin A1c, a subtype of glycosylated hemoglobin), and C peptides (a byproduct in the formation of insulin)
[79]
. In addition, chitosan oligosaccharide (GO2KA1) could improve the impaired glucose tolerance and fasting glucose levels in a crossover, randomized, controlled clinical trial
[68]
. A meta-analysis indicates that chitosan treatment for patients significantly decreases DBP (diastolic blood pressure) in short-term interventions
[80]
.
References
- Thadathil, N.; Velappan, S.P. Recent developments in chitosanase research and its biotechnological applications: A review. Food Chem. 2014, 150, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Sun, T.; Zhong, R.; Ma, L.; You, C.; Tian, M.; Li, H.; Wang, C. Effects of Chitosan Oligosaccharides on Human Blood Components. Front. Pharmacol. 2018, 9, 1412. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Wang, W.; Wang, S.; Zhang, L.; Guo, Y. An Overview of the Protective Effects of Chitosan and Acetylated Chitosan Oligosaccharides against Neuronal Disorders. Mar. Drugs 2017, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Bernaerts, K.V.; Dodi, G.; Shavandi, A. Chitooligosaccharides for wound healing biomaterials engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111266. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Brás, T.; Rosa, D.; Gonçalves, A.C.; Gomes, A.C.; Alves, V.D.; Crespo, J.G.; Duarte, M.F.; Neves, L.A. Development of bioactive films based on chitosan and Cynara cardunculus leaves extracts for wound dressings. Int. J. Biol. Macromol. 2020, 163, 1707–1718. [Google Scholar] [CrossRef]
- Wu, X.; Kim, M.J.; Yang, H.J.; Park, S. Chitosan alleviated menopausal symptoms and modulated the gut microbiota in estrogen-deficient rats. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, D.; Dutta, P.; Kalita, J.; Wann, S.B.; Manna, P. Chitosan: A promising therapeutic agent and effective drug delivery system in managing diabetes mellitus. Carbohydr. Polym. 2020, 247, 116594. [Google Scholar] [CrossRef]
- Belorkar, S.A.; Gupta, A.K. Oligosaccharides: A boon from nature’s desk. AMB Express 2016, 6, 82. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Ferket, P.R.; Hong, Q.H.; Zhou, J.; Cao, G.T.; Zhou, L.; Chen, A.G. Effect of chito-oligosaccharide on growth performance, intestinal barrier function, intestinal morphology and cecal microflora in weaned pigs. J. Anim. Sci. 2012, 90, 2671–2676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiao, S.; Wang, Z.A.; Du, Y. Exploring Effects of Chitosan Oligosaccharides on Mice Gut Microbiota in in vitro Fermentation and Animal Model. Front. Microbiol. 2018, 9, 2388. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, P.; Pourghassem Gargari, B.; Asghari Jafar-abadi, M. Oligofructose-enriched inulin improves some inflammatory markers and metabolic endotoxemia in women with type 2 diabetes mellitus: A randomized controlled clinical trial. Nutrition 2014, 30, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Azad, M.A.K.; Lin, Y.; Kim, S.W.; Tian, Y.; Liu, G.; Wang, H. Biological Effects and Applications of Chitosan and Chito-Oligosaccharides. Front. Physiol. 2019, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016, 92, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. CMLS 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Derrien, M.; van Passel, M.W.; van de Bovenkamp, J.H.; Schipper, R.G.; de Vos, W.M.; Dekker, J. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes 2010, 1, 254–268. [Google Scholar] [CrossRef]
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef]
- Ottman, N.; Davids, M.; Suarez-Diez, M.; Boeren, S.; Schaap, P.J.; Martins Dos Santos, V.A.P.; Smidt, H.; Belzer, C.; de Vos, W.M. Genome-Scale Model and Omics Analysis of Metabolic Capacities of Akkermansia muciniphila Reveal a Preferential Mucin-Degrading Lifestyle. Appl. Environ. Microbiol. 2017, 83, e01014-17. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Macchione, I.G.; Lopetuso, L.R.; Ianiro, G.; Napoli, M.; Gibiino, G.; Rizzatti, G.; Petito, V.; Gasbarrini, A.; Scaldaferri, F. Akkermansia muciniphila: Key player in metabolic and gastrointestinal disorders. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8075–8083. [Google Scholar] [PubMed]
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Ohnishi, H. Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 7381–7391. [Google Scholar] [CrossRef]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef]
- Levin, P.D.; Weissman, C. Obesity, metabolic syndrome, and the surgical patient. Med. Clin. N. Am. 2009, 93, 1049–1063. [Google Scholar] [CrossRef]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef]
- Jameson, J.L. Endocrinology: Adult and Pediatric, 7th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2016. [Google Scholar]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nature reviews. Endocrinology 2019, 15, 261–273. [Google Scholar]
- Dong, T.S.; Jacobs, J.P. Nonalcoholic fatty liver disease and the gut microbiome: Are bacteria responsible for fatty liver? Exp. Biol. Med. 2019, 244, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. North Am. 2014, 43, 1–23. [Google Scholar] [CrossRef]
- De la Cuesta-Zuluaga, J.; Mueller, N.T.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Metformin Is Associated With Higher Relative Abundance of Mucin-Degrading Akkermansia muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut. Diabetes Care 2017, 40, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.S.; Hoyte, C. Review of Biguanide (Metformin) Toxicity. J. Intensive Care Med. 2019, 34, 863–876. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; An, J.; Song, Y.; Lee, C.K.; Kim, K.; Kong, H. Alterations in Gut Microbiota by Statin Therapy and Possible Intermediate Effects on Hyperglycemia and Hyperlipidemia. Front. Microbiol. 2019, 10, 1947. [Google Scholar] [CrossRef]
- Khan, T.J.; Ahmed, Y.M.; Zamzami, M.A.; Siddiqui, A.M.; Khan, I.; Baothman, O.A.S.; Mehanna, M.G.; Kuerban, A.; Kaleemuddin, M.; Yasir, M. Atorvastatin Treatment Modulates the Gut Microbiota of the Hypercholesterolemic Patients. Omics J. Integr. Biol. 2018, 22, 154–163. [Google Scholar] [CrossRef]
- Li, S.; Yu, Y.; Jin, Z.; Dai, Y.; Lin, H.; Jiao, Z.; Ma, G.; Cai, W.; Han, B.; Xiang, X. Prediction of pharmacokinetic drug-drug interactions causing atorvastatin-induced rhabdomyolysis using physiologically based pharmacokinetic modelling. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 119, 109416. [Google Scholar] [CrossRef]
- Watanabe, K.; Oda, S.; Matsubara, A.; Akai, S.; Yokoi, T. Establishment and characterization of a mouse model of rhabdomyolysis by coadministration of statin and fibrate. Toxicol. Lett. 2019, 307, 49–58. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, F.; Yan, Y.; Zhang, Z.; Wang, L.; Qin, C. Lipid-lowering activities of chitosan and its quaternary ammonium salt for the hyperlipidemia rats induced by high-fat diets. Int. J. Biol. Macromol. 2019, 132, 922–928. [Google Scholar] [CrossRef]
- Luan, F.; Wei, L.; Zhang, J.; Tan, W.; Chen, Y.; Dong, F.; Li, Q.; Guo, Z. Preparation and Characterization of Quaternized Chitosan Derivatives and Assessment of Their Antioxidant Activity. Molecules 2018, 23, 516. [Google Scholar] [CrossRef]
- Guan, G.; Wang, H.; Chen, S.; Liu, G.; Xiong, X.; Tan, B.; Duraipandiyan, V.; Al-Dhabi, N.A.; Fang, J. Dietary Chitosan Supplementation Increases Microbial Diversity and Attenuates the Severity of Citrobacter rodentium Infection in Mice. Mediat. Inflamm. 2016, 2016, 9236196. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, J.; Cao, P.; Pan, H.; Ding, C.; Xiao, T.; Zhang, P.; Guo, J.; Su, Z. Anti-obese effect of glucosamine and chitosan oligosaccharide in high-fat diet-induced obese rats. Mar. Drugs 2015, 13, 2732–2756. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Anaya, L.M.; Soltero, J.F.; Rinaudo, M. DNA/chitosan electrostatic complex. Int. J. Biol. Macromol. 2016, 88, 345–533. [Google Scholar] [CrossRef]
- Ahmed, K.F.; Aschi, A.; Nicolai, T. Formation and characterization of chitosan-protein particles with fractal whey protein aggregates. Colloids Surf. B Biointerfaces 2018, 196, 257–264. [Google Scholar] [CrossRef]
- Liu, S.H.; Chiu, C.Y.; Shi, C.M.; Chiang, M.T. Functional Comparison of High and Low Molecular Weight Chitosan on Lipid Metabolism and Signals in High-Fat Diet-Fed Rats. Mar. Drugs 2018, 16, 251. [Google Scholar] [CrossRef]
- Dimzon, I.K.; Ebert, J.; Knepper, T.P. The interaction of chitosan and olive oil: Effects of degree of deacetylation and degree of polymerization. Carbohydr. Polym. 2013, 92, 564–570. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef]
- Michos, E.D.; McEvoy, J.W.; Blumenthal, R.S. Lipid Management for the Prevention of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2019, 16, 1557–1567. [Google Scholar] [CrossRef]
- [35]
- Rizzo, M.; Giglio, R.V.; Nikolic, D.; Patti, A.M.; Campanella, C.; Cocchi, M.; Katsiki, N.; Montalto, G. Effects of chitosan on plasma lipids and lipoproteins: A 4-month prospective pilot study. Angiology 2014, 65, 538–542. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Mondragon, A.D.C.; Lamas, A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Animal-Origin Prebiotics Based on Chitin: An Alternative for the Future? A Critical Review. Foods 2020, 9, 782. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. The AMP-activated protein kinase pathway--new players upstream and downstream. J. Cell Sci. 2004, 117, 5479–5487. [Google Scholar] [CrossRef] [PubMed]
- Clarke, P.R.; Hardie, D.G. Regulation of HMG-CoA reductase: Identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1990, 9, 2439–2446. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.A.; Pinkosky, S.L.; Filippov, S.; Hanselman, J.C.; Cramer, C.T.; Newton, R.S. AMP-activated protein kinase: An emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J. Lipid Res. 2012, 53, 2490–2514. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Ge, H.; Yang, G.; Huang, L.; Motola, D.L.; Pourbahrami, T.; Li, C. Oligomerization and regulated proteolytic processing of angiopoietin-like protein 4. J. Biol. Chem. 2004, 279, 2038–2045. [Google Scholar] [CrossRef]
- Lei, X.; Shi, F.; Basu, D.; Huq, A.; Routhier, S.; Day, R.; Jin, W. Proteolytic processing of angiopoietin-like protein 4 by proprotein convertases modulates its inhibitory effects on lipoprotein lipase activity. J. Biol. Chem. 2011, 286, 15747–15756. [Google Scholar] [CrossRef]
- Yin, W.; Romeo, S.; Chang, S.; Grishin, N.V.; Hobbs, H.H.; Cohen, J.C. Genetic variation in ANGPTL4 provides insights into protein processing and function. J. Biol. Chem. 2009, 284, 13213–13222. [Google Scholar] [CrossRef]
- Ge, H.; Cha, J.Y.; Gopal, H.; Harp, C.; Yu, X.; Repa, J.J.; Li, C. Differential regulation and properties of angiopoietin-like proteins 3 and 4. J. Lipid Res. 2005, 46, 1484–1490. [Google Scholar] [CrossRef]
- Davies, B.S.; Beigneux, A.P.; Barnes, R.H., 2nd; Tu, Y.; Gin, P.; Weinstein, M.M.; Nobumori, C.; Nyrén, R.; Goldberg, I.; Olivecrona, G.; et al. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 2010, 12, 42–52. [Google Scholar] [CrossRef]
- Kersten, S.; Lichtenstein, L.; Steenbergen, E.; Mudde, K.; Hendriks, H.F.; Hesselink, M.K.; Schrauwen, P.; Müller, M. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 969–974. [Google Scholar] [CrossRef]
- González-Muniesa, P.; de Oliveira, C.; Pérez de Heredia, F.; Thompson, M.P.; Trayhurn, P. Fatty acids and hypoxia stimulate the expression and secretion of the adipokine ANGPTL4 (angiopoietin-like protein 4/fasting-induced adipose factor) by human adipocytes. J. Nutr. Nutr. 2011, 4, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chiu, T.Y.; Liang, Y.J.; Lee, C.J.; Liu, C.S.; Suen, C.S.; Yen, J.J.; Chen, H.T.; Hwang, M.J.; Hussain, M.M.; et al. PRAP1 is a novel lipid binding protein that promotes lipid absorption by facilitating MTTP-mediated lipid transport. J. Biol. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Matsumoto, H.; Kennedy, S.; Newberry, E.P.; Moritz, W.; DeBosch, B.J.; Moley, K.H.; Rubin, D.C.; Warner, B.W.; Kau, A.L.; et al. Impaired Chylomicron Assembly Modifies Hepatic Metabolism Through Bile Acid-Dependent and Transmissible Microbial Adaptations. Hepatology 2019, 70, 1168–1184. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nature reviews. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42 (Suppl. 1), S13–S28. [Google Scholar] [CrossRef]
- Jeong, S.; Min Cho, J.; Kwon, Y.I.; Kim, S.C.; Yeob Shin, D.; Ho Lee, J. Chitosan oligosaccharide (GO2KA1) improves postprandial glycemic response in subjects with impaired glucose tolerance and impaired fasting glucose and in healthy subjects: A crossover, randomized controlled trial. Nutr. Diabetes 2019, 9, 31. [Google Scholar] [CrossRef]
- Hers, I.; Vincent, E.E.; Tavaré, J.M. Akt signalling in health and disease. Cell. Signal. 2011, 23, 1515–1527. [Google Scholar] [CrossRef]
- Liu, T.Y.; Shi, C.X.; Gao, R.; Sun, H.J.; Xiong, X.Q.; Ding, L.; Chen, Q.; Li, Y.H.; Wang, J.J.; Kang, Y.M.; et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin. Sci. 2015, 129, 839–850. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Zeng, Y.; Huang, D.; Xu, Q. Involvement of AMPK activation in the inhibition of hepatic gluconeogenesis by Ficus carica leaf extract in diabetic mice and HepG2 cells. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 109, 188–194. [Google Scholar] [CrossRef]
- Liu, S.H.; Chang, Y.H.; Chiang, M.T. Chitosan reduces gluconeogenesis and increases glucose uptake in skeletal muscle in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2010, 58, 5795–5800. [Google Scholar] [CrossRef]
- Catrysse, L.; van Loo, G. Inflammation and the Metabolic Syndrome: The Tissue-Specific Functions of NF-κB. Trends Cell Biol. 2017, 27, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Kunanusornchai, W.; Witoonpanich, B.; Tawonsawatruk, T.; Pichyangkura, R.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide suppresses synovial inflammation via AMPK activation: An in vitro and in vivo study. Pharmacol. Res. 2016, 113, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.M.; Aronoff, D.M. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin. Microbiol. Infect. 2016, 22, 178.e1–178.e9. [Google Scholar] [CrossRef] [PubMed]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in cancer: A review. J. Cell. Physiol. 2019, 235, 5683–5699. [Google Scholar] [CrossRef] [PubMed]
- Tai, T.S.; Sheu, W.H.; Lee, W.J.; Yao, H.T.; Chiang, M.T. Effect of chitosan on plasma lipoprotein concentrations in type 2 diabetic subjects with hypercholesterolemia. Diabetes Care 2000, 23, 1703–1704. [Google Scholar] [CrossRef]
- Kim, H.J.; Ahn, H.Y.; Kwak, J.H.; Shin, D.Y.; Kwon, Y.I.; Oh, C.G.; Lee, J.H. The effects of chitosan oligosaccharide (GO2KA1) supplementation on glucose control in subjects with prediabetes. Food Funct. 2014, 5, 2662–2669. [Google Scholar] [CrossRef]
- Huang, H.; Zou, Y.; Chi, H. Quantitative assessment of the effects of chitosan intervention on blood pressure control. Drug Des. Dev. Ther. 2018, 12, 67–75. [Google Scholar] [CrossRef]
