Tissue renin-angiotensin system (tRAS) is involved in the progression of various human diseases. This system contains two regulatory pathways: a pathological pro-inflammatory pathway containing the Angiotensin Converting Enzyme (ACE)/Angiotensin II (AngII)/Angiotensin II receptor type 1 (AGTR1) axis and a protective anti-inflammatory pathway involving the Angiotensin II receptor type 2 (AGTR2)/ACE2/Ang1–7/MasReceptor axis.
- renin-angiotensin
- RAS
- senescence
- cardiovascular
- vulvodynia
- intervertebral disc
- inflammation
- regeneration
- COVID-19
1. Introduction
The classic renin-angiotensin-aldosterone system (RAAS) is a well-known regulator of salt and water homeostasis. For a long time, the RAAS was viewed as an endocrine system in which kidney cells convert the blood’s prorenin to renin and secrete it into circulation. In this classical point of view, the plasma renin itself converts the angiotensinogen, secreted by the liver, to angiotensin I, which is then converted by angiotensin-converting enzyme (ACE) on the surface of vascular endothelial cells to angiotensin II (AngII). This circulating Angiotensin II can now bind onto blood vessel cells to reveal vasoconstrictive effects. Further, AngII stimulates aldosterone secretion by zona glomerulosa cells of adrenal glands, which increases sodium and water retention in kidneys, leading to an increase in blood pressure. As the current review focuses on local RAS effects in various tissues, we will continue using RAS instead of RAAS, although these terms are often used interchangeably. Nearly a hundred years after the first description of the circulating renin-angiotensin system by Tigerstedt and Bergmann, evidence has arisen that local renin-angiotensin systems are present in multiple human tissues [1][2].
2. RTAS and Relevant Intracellular Processes
This complex system seems to be independent of the circulating renin-angiotensin-aldosterone system, is found intracellularly, and interacts with numerous relevant intracellular processes (Figure 1).
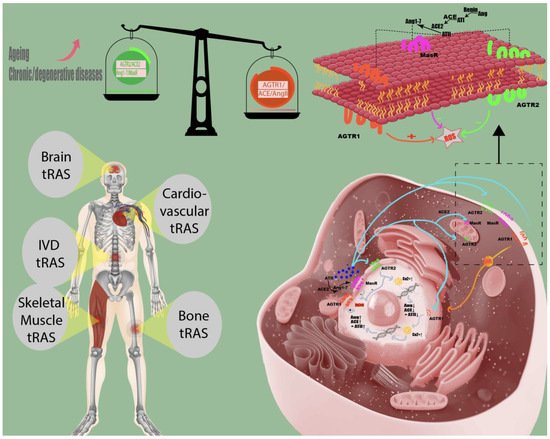
Figure 1. Tissue renin-angiotensin system in humans. Local renin-angiotensin systems are found in numerous human tissues, including skeletal muscle, bone, cardiovascular, brain, and intervertebral disc (IVD) tissue. The angiotensin II receptor type 2 (AGTR2) and the Ang1–7/Mas receptor (MasR) axis counteract the pro-inflammatory effects (increasing intracellular reactive oxygen species (ROS) of accumulating angiotensin II concentrations (AngII) on angiotensin II receptor type 1 (AGTR1). Chronic/degenerative disease states are characterized by the domination of the pathological tRAS pathway (angiotensin-converting enzyme (ACE)/AngII/AGTR1) over the protective axis (AGTR2/ACE2/Ang1–7/MasR), and aging might shift the balance between protective and pathological RAS axis towards AGTR1/ACE/AngII [2][3].
Interestingly, components of the renin-angiotensin system were found in primitive chordates and tunicates before the circulating RAS had reached its full development, indicating ancestral functions outside of their roles in regulating salt and water homeostasis [4]. Furthermore, renin-like activities were found in different tissues, emphasizing the RAS effectors’ local production and independence from the available circulatory RAS effectors [5]. However, it seems impossible to distinguish the in vivo effects from the circulatory RAS, as there may be multiple interactions with the available circulatory peptides of the RAS.
3. Two Regulatory Pathways
The complex mechanisms of the tissue RAS (tRAS) can be simplified into two regulatory pathways: a pathological pro-inflammatory pathway containing the ACE/AngII/angiotensin II type 1 receptor (AGTR1) axis and a protective anti-inflammatory pathway involving the angiotensin II type 2 receptor (AGTR2)/ACE2/Ang1–7/MasReceptor axis. Its main effector, angiotensin II, a well-known vasoconstrictor, is a double-edged sword that can negatively affect tissues when stimulating the pathological pathway or have positive effects when stimulating the AGTR2 [6].
Another angiotensin II pathway is the conversion to Ang1–7 through angiotensin-converting enzyme 2 (ACE2), leading to positive effects by stimulating the Ang1–7/MasReceptor axis.
The role of angiotensin II in most human tissues remains largely unknown. Should it be considered as an intracrine, autocrine, or paracrine hormone? Based on previous findings, AngII can be internalized through AGTR1 incorporation, and the intracellular concentration is strictly regulated by complex intercellular interactions involving multiple cellular compartments, including the nucleus and mitochondria [7][8][9]. Therefore, it appears likely that cells do not only directly react to changes in tissue angiotensin II through the activation of surface receptors, but they are also actively involved in regulating the tissue angiotensin II concentration through 1) AGTR1 mediated internalization, 2) conversion through ACE2 and 3) secretion of intracellularly produced angiotensin II [8][10]. This implies that angiotensin II is an intracrine, autocrine, and paracrine hormone at the same time.
For a long time, it has been a general consensus that AGTR2, in contrast to AGTR1, is primarily expressed during fetal development and is less abundant in adult tissues unless it gets somehow reactivated [11]. This implicated that its impact as part of the protective RAS axis in adult tissue is negligible. The evidence leading to this consensus was primarily provided by studies in rodent animals almost 20 years ago, conducting in situ hybridization techniques, autoradiography, and ligand binding studies to investigate the tissue-specific expression during the oncogenic stages [11]. At the same time, other authors were stating the contrary by revealing a significant expression of the AT2 receptor in adult rodent animals [12][13]. More recent research provides convincing data, including data in humans, contrary to the dogma that AT2 receptors are primarily expressed in fetal tissues, showing its significant expression in adult bone, bone marrow mesenchymal stem cells, synovial cells, fibroblasts, heart, kidney, adrenal gland, uterus, pancreas, retina, skin, smooth muscle cells of vasculature, and intervertebral disc cells [2][14][15][16][17][18][19][20]. Notably, some tissues’ expression levels can change depending on pathological states and tissue remodeling processes [19]. As most of the research on AT2 receptor changes during development was conduct in rodent animals, it is unclear how and why its expression may vary in humans in different developmental stages. The dogma stating AT2 receptors are primarily important in an early stage of life should be revisited based on the current evidence. More research should be conduct on its impact and the variable expression levels in adult human tissues.
It remains an open question about which factors define whether Ang II acts positively or negatively. It is currently suggested that the protective pathway’s variable expression level is the leading factor that defines the impact of AngII in the tissues [21]. Therefore, it is believed that an imbalance between these regulatory pathways could directly impact multiple cell processes, including inflammation, immune activity, and cell senescence [22][23][24].
Nevertheless, angiotensin II is not the only molecule of interest for the local renin-angiotensin system. Little is known about the receptors and effectors of this system’s protective axis and their therapeutic relevance. An overwhelming number of recent studies indicated that its beneficial components might save the tissue from the inflammatory and tissue-degenerative consequences of increased angiotensin II tissue concentrations [6][25][26][27]. To date, most studies have focused on the pathological pathway of tRAS and proposed positive effects on cell and tissue levels following the subsequent inhibition of the pathological pathway.
References
- Tigerstedt, R.; Bergman, P.Q. Niere und kreislauf. Skand. Archiv Physiol. 1898, 8, 223–271.
- Paul, M.; Poyan Mehr, A.; Kreutz, R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006, 86, 747–803.
- Yoon, H.E.; Kim, E.N.; Kim, M.Y.; Lim, J.H.; Jang, I.-A.; Ban, T.H.; Shin, S.J.; Park, C.W.; Chang, Y.S.; Choi, B.S. Age-associated changes in the vascular renin-angiotensin system in mice. Oxid. Med. Cell Longev. 2016, 2016, 6731093.
- Fournier, D.; Luft, F.C.; Bader, M.; Ganten, D.; Andrade-Navarro, M.A. Emergence and evolution of the renin-angiotensin-aldosterone system. J. Mol. Med. 2012, 90, 495–508.
- Nehme, A.; Zouein, F.A.; Deris Zayeri, Z.; Zibara, K. An update on the tissue renin angiotensin system and its role in physiology and pathology. J. Cardiovasc. Dev. Dis. 2019, 6, 14.
- Unger, T.; Steckelings, U.M.; dos Santos, R.S. The Protective Arm of the Renin Angiotensin: Functional Aspects and Therapeutic Implications; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-0-12-801485-1.
- Villar-Cheda, B.; Costa-Besada, M.A.; Valenzuela, R.; Perez-Costas, E.; Melendez-Ferro, M.; Labandeira-Garcia, J.L. The intracellular angiotensin system buffers deleterious effects of the extracellular paracrine system. Cell Death Dis. 2017, 8, e3044.
- Jan Danser, A.H. Local renin–angiotensin systems: The unanswered questions. Int. J. Biochem. Cell Biol. 2003, 35, 759–768.
- Filipeanu, C.M.; Henning, R.H.; Nelemans, S.A.; de Zeeuw, D. Review: Intracellular angiotensin II: From myth to reality? J. Renin. Angiotensin. Aldosterone Syst. 2001, 2, 219–226.
- Ferrario, C.M.; Ahmad, S.; Varagic, J.; Cheng, C.P.; Groban, L.; Wang, H.; Collawn, J.F.; Dell′Italia, L.J. Intracrine angiotensin II functions originate from noncanonical pathways in the human heart. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H404–H414.
- de Gasparo, M.; Catt, K.J.; Inagami, T.; Wright, J.W.; Unger, T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 2000, 52, 415–472.
- Cao, Z.; Kelly, D.J.; Cox, A.; Casley, D.; Forbes, J.M.; Martinello, P.; Dean, R.; Gilbert, R.E.; Cooper, M.E. Angiotensin Type 2 receptor is expressed in the adult rat kidney and promotes cellular proliferation and apoptosis. Kidney Int. 2000, 58, 2437–2451.
- Lenkei, Z.; Palkovits, M.; Corvol, P.; Llorens-Cortes, C. Distribution of angiotensin ii type-2 receptor (AT2) MRNA expression in the adult rat brain. J. Comp. Neurol. 1996, 373, 322–339.
- Yu, L.; Zheng, M.; Wang, W.; Rozanski, G.J.; Zucker, I.H.; Gao, L. Developmental changes in AT1 and AT2 receptor-protein expression in rats. J. Renin. Angiotensin. Aldosterone Syst. 2010, 11, 214–221.
- Terenzi, R.; Manetti, M.; Rosa, I.; Romano, E.; Galluccio, F.; Guiducci, S.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Angiotensin II type 2 receptor (AT2R) as a novel modulator of inflammation in rheumatoid arthritis synovium. Sci. Rep. 2017, 7, 13293.
- Xu, X.; He, H.; Hu, S.; Han, J.; Huang, L.; Xu, J.; Xie, J.; Liu, A.; Yang, Y.; Qiu, H. Ang II-AT2R increases mesenchymal stem cell migration by signaling through the FAK and RhoA/Cdc42 pathways in vitro. Stem. Cell Res. Ther. 2017, 8, 164.
- Izu, Y.; Mizoguchi, F.; Kawamata, A.; Hayata, T.; Nakamoto, T.; Nakashima, K.; Inagami, T.; Ezura, Y.; Noda, M. Angiotensin II type 2 receptor blockade increases bone mass. J. Biol. Chem. 2009, 284, 4857–4864.
- Galindo, M.; Santiago, B.; Palao, G.; Gutierrez-Cañas, I.; Ramirez, J.C.; Pablos, J.L. Coexpression of AT1 and AT2 receptors by human fibroblasts is associated with resistance to angiotensin II. Peptides 2005, 26, 1647–1653.
- Jones, E.S.; Vinh, A.; McCarthy, C.A.; Gaspari, T.A.; Widdop, R.E. AT2 Receptors: Functional relevance in cardiovascular disease. Pharmacol. Ther. 2008, 120, 292–316.
- Li, Z.; Wystrach, L.; Bernstein, A.; Grad, S.; Alini, M.; Richards, R.; Kubosch, D.; Südkamp, N.; Izadpanah, K.; Kubosch, E.; et al. The tissue-renin-angiotensin-system of the human intervertebral disc. eCM 2020, 40, 115–132.
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020, 126, 1456–1474.
- Mogi, M. Effect of renin–angiotensin system on senescence. Geriatr. Gerontol. Int. 2020, 20, 520–525.
- Satou, R.; Penrose, H.; Navar, L.G. Inflammation as a regulator of the renin-angiotensin system and blood pressure. Curr. Hypertens Rep. 2018, 20, 100.
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol. Med. 2010, 2, 247–257.
- Stone, R.E.; Liu, S.; Levy, A.M.; Kashani, N.; Louie, S.G.; Rodgers, K.E.; Kelland, E.E.; Lund, B.T. Activation of the protective arm of the renin angiotensin system in demyelinating disease. J. Neuroimmune. Pharmacol. 2020, 15, 249–263.
- Soto, M.; Delatorre, N.; Hurst, C.; Rodgers, K.E. Targeting the protective arm of the renin-angiotensin system to reduce systemic lupus erythematosus related pathologies in MRL-Lpr mice. Front. Immunol. 2020, 11, 1572.
- Namsolleck, P.; Moll, G.N. Does activation of the protective renin-angiotensin system have therapeutic potential in COVID-19? Mol. Med. 2020, 26, 80.
