Lung cancer is the second most common cancer and remains the leading cause of cancer-related mortality in the U.S. Non-small-cell lung cancer (NSCLC) accounts for 84% of lung cancer cases. Our previous study found that zinc finger protein 71 (ZNF71) mRNA expression was associated with chemosensitivity and its protein expression was prognostic of non-small-cell lung cancer (NSCLC).
- KRAB isoform
- zinc finger protein
- EMT
- prognosis
- chemoresponse
- proliferation
- CRISPR-Cas9
- RNAi
1. Introduction
Lung cancer is the second most common cancer and remains the leading cause of cancer-related mortality in the U.S. Non-small-cell lung cancer (NSCLC) accounts for 84% of lung cancer cases. Major histological subtypes of NSCLC include lung adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. In 2020, an estimated 228,820 adults (116,300 men and 112,520 women) in the US were diagnosed with lung cancer [1]. About 30% to 55% of the patients with NSCLC develop recurrence and die of the disease within 5 years of the surgical removal of their tumors [2]. According to the current practice guidelines, NSCLC patients with stage 2 and above receive chemotherapy, with additional radiation for stage 3A patients [3]. While adjuvant chemotherapy of stage 2 and stage 3 disease has resulted in 10–15% increase in overall survival [4], the prognosis for early-stage NSCLC remains poor [5], indicating that some patients may not benefit from it. To date, physicians do not have a precise tool to identify patients with resectable NSCLC that are likely to develop tumor recurrence or metastasis. Our previous study developed a 7-gene assay for NSCLC prognosis and prediction of chemotherapeutic benefits [6]. The ability of this gene assay to identify those at a high risk for recurrence or metastasis would potentially inform selection of specific adjuvant chemotherapy for these patients.
Among this 7-gene signature, mRNA expression of zinc finger protein 71 (
ZNF71
) was positively associated with survival in patients who received cisplatin, carboplatin, and Taxol in the studied cohorts, indicating its association with chemosensitivity [6]. Although
ZNF71
mRNA expression was not associated with NSCLC survival in the overall patient cohorts analyzed in qRT-PCR, higher ZNF71 protein expression quantified with AQUA was associated with a more favorable survival outcome in two separate NSCLC cohorts (
n = 291) using tissue microarrays (TMA) [6]. Zinc finger proteins (ZNFs) are involved in DNA repair, degradation of proteins, signal transductions, migration of cells, regulation of apoptosis, lipid binding, and transcription regulation [7][8]. The Krüppel associated box (KRAB) is a transcriptional repression domain and is commonly present in human zinc finger protein-based transcription factors, i.e., KRAB zinc finger proteins (KRAB-ZFPs) [9][10][11]. Transcriptional repression mediated by KRAB-ZFPs is linked to cell proliferation, differentiation, apoptosis, and cancer [12]. ZNF71 (EZFIT) was first identified by Mataki et al. [13] as a ZFP induced by tumor necrosis factor α (TNF-α) in human umbilical vein endothelial cells. Single-nucleotide polymorphisms of ZNF71 were found to be linked with total serum IgE in Korean asthmatics in a genome-wide association study [14]. To the best of our knowledge, there has been no report on molecular analysis of ZNF71 KRAB isoform in cancer.
= 291) using tissue microarrays (TMA) [6]. Zinc finger proteins (ZNFs) are involved in DNA repair, degradation of proteins, signal transductions, migration of cells, regulation of apoptosis, lipid binding, and transcription regulation [7,8]. The Krüppel associated box (KRAB) is a transcriptional repression domain and is commonly present in human zinc finger protein-based transcription factors, i.e., KRAB zinc finger proteins (KRAB-ZFPs) [9,10,11]. Transcriptional repression mediated by KRAB-ZFPs is linked to cell proliferation, differentiation, apoptosis, and cancer [12]. ZNF71 (EZFIT) was first identified by Mataki et al. [13] as a ZFP induced by tumor necrosis factor α (TNF-α) in human umbilical vein endothelial cells. Single-nucleotide polymorphisms of ZNF71 were found to be linked with total serum IgE in Korean asthmatics in a genome-wide association study [14]. To the best of our knowledge, there has been no report on molecular analysis of ZNF71 KRAB isoform in cancer.
Epithelial-to-mesenchymal transition (EMT) is a highly dynamic process in which epithelial cells can convert to a mesenchymal phenotype. EMT is also reversible by the mesenchymal-to-epithelial transition (MET). Emerging evidence reveals the involvement of EMT in tumor progression, metastasis, and resistance to cancer treatment [15][16][17][18]. However, the involvement of EMT in cancer patient outcomes remains controversial [19]. Recent studies have evaluated EMT as well as stromal and immune infiltration in tumors using transcriptional profiles [19][20][21]. This study sought to investigate the expression of
Epithelial-to-mesenchymal transition (EMT) is a highly dynamic process in which epithelial cells can convert to a mesenchymal phenotype. EMT is also reversible by the mesenchymal-to-epithelial transition (MET). Emerging evidence reveals the involvement of EMT in tumor progression, metastasis, and resistance to cancer treatment [15,16,17,18]. However, the involvement of EMT in cancer patient outcomes remains controversial [19]. Recent studies have evaluated EMT as well as stromal and immune infiltration in tumors using transcriptional profiles [19,20,21]. This study sought to investigate the expression of
ZNF71 KRAB
and
KRAB
-less isoforms in NSCLC tumors and cell lines and their association with patient outcome and chemotherapeutic response. Tripartite motif containing 28 (TRIM28) protein, a universal co-factor for KRAB-ZFP transcription factors [22], contributes to EMT and might be important for tumor metastasis in lung cancer [23]. Based on the link between KRAB-ZFPs and EMT [24], this study sought to explore whether NSCLC tumors with different EMT characteristics have a distinct survival outcome and the association of ZNF71 isoforms and EMT. Molecular functions of ZNF71 in NSCLC proliferation were evaluated with CRISPR-Cas9 and RNAi approaches. The overall study scheme is provided in
.
Figure 1.
Specifically, mRNA expression of
ZNF71
isoforms and their prognostic implications were analyzed with public RNA-seq data of NSCLC patient tumors (
n
= 197) [25] and cell lines (
n
= 117) [26]. A 14-gene EMT classifier was constructed to evaluate the hybrid EMT states in NSCLC tumors, and
ZEB1
expression was used to evaluate the EMT states in cell lines. This EMT classification was further validated using stromal infiltration scores computed with software ESTIMATE [20]. The association between ZNF71 overall and isoform expression and EMT was examined in NSCLC tumors and cell lines. Functional assessment of ZNF71 in NSCLC proliferation was evaluated with public CRISPR-Cas9 [27] and RNAi [28] screening data. Finally, the association of the ZNF71 isoform and overall expression and chemoresponse to nine drugs commonly used to treat NSCLC was examined in cell lines (
).
2. Expression of ZNF71 Isoforms in NSCLC Tumors and Cell Lines
ZNF71 gene is comprised of four exons, where exons 1 and 2 code for 180nt 5′UTR and the first 11 amino acids of the protein. Exon 3 codes for the next 43 amino acids (aa), encompassing most of the KRAB repression domain. Exon 4, the longest, codes for the remainder of the protein, including the 13-zinc-finger putative DNA binding domain and the predicted ~4 kb 3′UTR. The third KRAB-domain-containing exon could be alternatively spliced out to produce a KRAB-less isoform that will produce a shorter in-frame protein encoded by the last exon, i.e., exon 4. Approximately half of 800 human C2H2-type ZNF genes contain the evolutionary conserved one-exon-encoded KRAB domain, which could be alternatively spiced [9][10][11][9,10,11]. We analyzed ZNF71 isoform expression in the RNA-seq dataset GSE81089 of NSCLC tumor samples (n = 197) [25] and correlated the analysis with patient outcomes. Patient clinical characteristics is provided in Table A1. (supplementary could be found in https://www.mdpi.com/1422-0067/22/7/3752/htm)
The ZNF71 KRAB isoform (ZNF71_203_ENST00000599599) had a significantly higher expression (p < 0.001, t-tests) than the KRAB-less isoform (ZNF71_201_ENST00000328070) in NSCLC patient tumor samples (n = 197; Figure 2A) and cell lines (n = 117; Figure 2B). The expression of ZNF71 KRAB and KRAB-less isoforms was significantly correlated in both patient tumor samples (p < 8.6 × 10−15, Pearson’s correlation, Figure 2C) and cell lines (p < 2.3 × 10−8, Pearson’s correlation, Figure 2D). The expression of overall ZNF71 and its isoforms was not significantly different among different histological subtypes in patient tumors (ANOVA tests; Figure A1). The expression of ZNF71 overall and the KRAB isoform was significantly different among histological subtypes in NSCLC cell lines (p < 0.05, ANOVA tests; Figure A2). In the studied cell lines, large cell carcinoma had the highest ZNF71 overall expression. Adenosquamous carcinoma had the highest ZNF71 KRAB expression. Squamous cell carcinoma had the lowest expression of both ZNF71 overall and KRAB isoform (Figure A2).
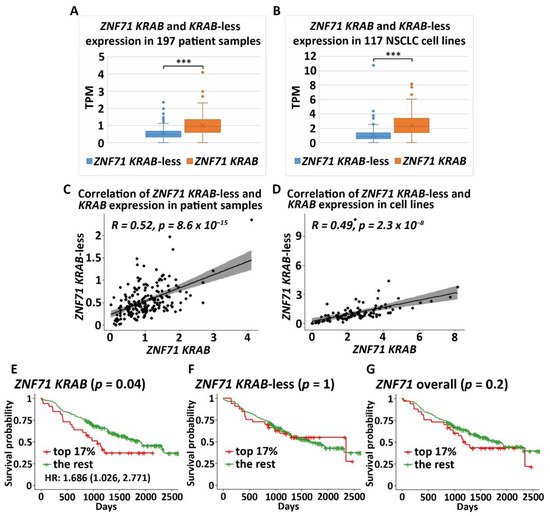
Figure 2.
ZNF71 KRAB
KRAB
A
ZNF71 KRAB
KRAB
p
t
B
ZNF71 KRAB
KRAB
p
t
C
ZNF71 KRAB
KRAB
D
ZNF71 KRAB
KRAB
E–G
ZNF71 KRAB
E
ZNF71 KRAB
F
ZNF71
G
ZNF71 KRAB
To evaluate the prognostic performance of ZNF71 KRAB, patient tumor samples were divided into two groups using a cutoff of ZNF71 KRAB expression level measured with transcripts per million (TPM) of 1.5, which corresponds to the top 17% of ZNF71 KRAB expression versus the rest in the patient cohort. The cutoff value of TPM of 1.5 was chosen because TPM values are commonly normalized to the value of either 1 or 2 in RNA-seq raw data processing. The results showed that when ZNF71 KRAB isoform was expressed higher, patients survived for a significantly shorter time (log-rank p = 0.04, Kaplan–Meier analysis; Figure 2E), with a hazard ratio of 1.686 [1.026, 2.771]. In contrast, ZNF71 overall expression or ZNF71 KRAB-less isoform expression did not generate significant prognostic stratification in the patient cohort using the same cutoff (top 17% expression level versus the rest) in Kaplan–Meier survival analyses (Figure 2F,G). These results indicate that the ZNF71 KRAB isoform is a more accurate prognostic factor for NSCLC than ZNF71 overall expression and the KRAB-less isoform.
3. Association of ZNF71 KRAB Isoform with EMT
The above RNA-seq results were confirmed in qRT-PCR assays, which showed higher ZNF71 KRAB expression than the KRAB-less isoform in NSCLC cell lines (Figure 3A). Unfortunately, the ZNF71 antibody (Abcam, Cambridge, UK; ab87250) used in our previous study [6] was discontinued. We tried several other commercially available ZNF71 antibodies, but none of them was able to detect ZNF71 protein in our panel of NSCLC cell lines (not shown). After overexpression, ZNF71 protein expression (GeneTex, Irwin, CA, USA; Cat. No. GTX116553) was observed in HEK-293T cells (Figure 3B) in Western blots. The EMT properties of the NSCLC cell lines were tested using 3 mesenchymal markers (ZEB1, VIM, and FN1) and 11 epithelial markers (CDH1, EPCAM, ESRP1, ESRP2, DDR1, CTNNB1, CD24, CLDN7, KRT8, KRT19, and RAB25) using Western blots (Figure 3B). These 14 EMT markers were used to build an EMT classifier to divide patient samples into four groups based on the rank of the average expression values of all markers (Figure 3C). The average expression of 3 mesenchymal markers and that of 11 epithelial markers were computed for each patient sample, respectively. Based on the average epithelial and mesenchymal expression rank, patient samples were categorized into four EMT phenotypes: ranked as top 50% in both mesenchymal and epithelial (named High expression overlap), ranked as top 50% in mesenchymal but bottom 50% in epithelial (named Mesenchymal), ranked as top 50% in epithelial but bottom 50% in mesenchymal (named Epithelial), and ranked as the bottom 50% in both mesenchymal and epithelial (named Low expression overlap). A heatmap of the expression of EMT markers in categorized patient samples is shown in Figure 3D.

Figure 3. ZNF71 isoforms in epithelial-to-mesenchymal transition (EMT) and patient survival. (A) Relative quantity of ZNF71, KRAB, and KRAB-less in qRT-PCR assays of NSCLC cell lines. (B) Western blots of EMT markers as well as endogenous ZNF71 and overexpressed ZNF71 in HEK-293T (top lanes). (C) EMT classification of NSCLC tumors based on the average expression rank of epithelial and mesenchymal markers. The table shows the final four phenotype categorization of patient samples. (D) The expression of 14 EMT markers in patient tumors grouped by four phenotypes defined in D. (E–I) Kaplan–Meier survival analyses of patient tumors grouped by four EMT phenotypes (E) and in each phenotype (F–I). The Epithelial phenotype had the best patient survival outcome, and the High expression overlap phenotype had the worst patient survival outcome; ZNF71 KRAB is a poor prognosis marker in both phenotypes.
. ZNF71 isoforms in epithelial-to-mesenchymal transition (EMT) and patient survival. (A) Relative quantity of ZNF71, KRAB, and KRAB-less in qRT-PCR assays of NSCLC cell lines. (B) Western blots of EMT markers as well as endogenous ZNF71 and overexpressed ZNF71 in HEK-293T (top lanes). (C) EMT classification of NSCLC tumors based on the average expression rank of epithelial and mesenchymal markers. The table shows the final four phenotype categorization of patient samples. (D) The expression of 14 EMT markers in patient tumors grouped by four phenotypes defined in D. (E–I) Kaplan–Meier survival analyses of patient tumors grouped by four EMT phenotypes (E) and in each phenotype (F–I). The Epithelial phenotype had the best patient survival outcome, and the High expression overlap phenotype had the worst patient survival outcome; ZNF71 KRAB is a poor prognosis marker in both phenotypes.
Patients defined by the four EMT phenotypes had significantly different disease-specific survival (log-rank
p
<0.01, Kaplan–Meier analysis). The patients in the High expression overlap group had the worst prognosis, with the shortest survival time, whereas the patients in the Epithelial group had the best prognosis, with the longest survival time (Figure 3E). The four EMT phenotypes were independent of patient cancer stage (
p
= 0.8569, chi-square tests; Table A2). The EMT phenotype was associated with
ZNF71 KRAB
expression (
p
= 0.0099, chi-square tests; Table A3). Within each EMT phenotype, we further performed a Kaplan–Meier survival analysis for the high
ZNF71 KRAB
isoform expression group (TPM ≥ 1.5) versus the low
ZNF71 KRAB
isoform expression group (TPM < 1.5) to assess the association of
ZNF71 KRAB
expression and patient survival in each EMT phenotype. In Epithelial and High expression overlap phenotypes, patients with higher KRAB expression had a significantly lower survival probability than those with lower KRAB expression (Figure 3H,I). In the Mesenchymal and Low expression overlap phenotypes, there was no significant difference in patient survival between the high and low KRAB expression groups (Figure 3F,G). In the Mesenchymal group, tumors with higher ZNF71 KRAB expression had a worse prognosis (no statistical significance; Figure 3F). In the Low expression overlap group, tumors with higher ZNF71 KRAB expression had a better prognosis (no statistical significance; Figure 3G). These results suggest that the
ZNF71 KRAB
isoform is a poor-prognosis marker for NSCLC tumors with high expression of epithelial markers but not for NSCLC with low expression of epithelial markers.
ZNF71
overall expression and the
ZNF71 KRAB
and
KRAB
-less expression were all significantly different among all four EMT phenotypes in NSCLC tumors (
p
< 0.01, ANOVA tests; Figure 4A–C). Furthermore, Tukey’s honestly significant difference post-hoc test was performed among the EMT phenotypes. The expression of
ZNF71
(overall) and its isoforms was significantly lower in the Low expression overlap patient group than in the Epithelial phenotype, and the expression of
ZNF71
(overall) and the
KRAB
isoform was also significantly lower in the Low expression overlap patient group than in the High expression overlap patient group (
p
< 0.01, Tukey’s tests; Figure 4A–C). In Epithelial and High expression overlap phenotypes with higher expression of epithelial markers, the percentage of
ZNF71 KRAB
isoform high expression (TPM > 1.5) was greater than that in the other two EMT phenotypes (Figure 4D).
Solid tumors contain both epithelial and stromal cells, complicating the analysis of gene expression in patient tumor samples. On the contrary, NSCLC cell lines are devoid of any stromal components. Median ZEB1 gene expression was used to categorize a panel of NSCLC cell lines (n = 117) [26] into two groups: ZEB1 bottom 50% (more epithelial) and ZEB1 top 50% (more mesenchymal) [19]. The NSCLC cell lines with higher ZEB1 expression (ZEB1 top 50%) also had significantly higher expression of ZNF71 overall, KRAB, and KRAB-less isoforms compared to the cell lines that had lower ZEB1 expression (p < 0.05, two-sample t-tests; Figure 4E). These more mesenchymal cell lines would NOT be comparable to patient tumors with the Epithelial phenotype. A negative association of ZNF71 KRAB expression and patient survival was observed in NSCLC tumors with the Epithelial phenotype (Figure 3H).
Solid tumors contain both epithelial and stromal cells, complicating the analysis of gene expression in patient tumor samples. On the contrary, NSCLC cell lines are devoid of any stromal components. Median ZEB1 gene expression was used to categorize a panel of NSCLC cell lines (n = 117) [26] into two groups: ZEB1 bottom 50% (more epithelial) and ZEB1 top 50% (more mesenchymal) [19]. The NSCLC cell lines with higher ZEB1 expression (ZEB1 top 50%) also had significantly higher expression of ZNF71 overall, KRAB, and KRAB-less isoforms compared to the cell lines that had lower ZEB1 expression (p < 0.05, two-sample t-tests; Figure 4E). These more mesenchymal cell lines would NOT be comparable to patient tumors with the Epithelial phenotype. A negative association of ZNF71 KRAB expression and patient survival was observed in NSCLC tumors with the Epithelial phenotype (Figure 3H).

Figure 4. Association of ZNF71 isoforms and EMT in NSCLC tumors and cell lines. (
Association of ZNF71 isoforms and EMT in NSCLC tumors and cell lines. (
A
–
C) Comparison of ZNF71 overall (
) Comparison of ZNF71 overall (
A), KRAB-less isoform (
), KRAB-less isoform (
B), and KRAB isoform (
), and KRAB isoform (
C
) expression in patient tumors categorized by four EMT phenotypes. (
D) Distribution of ZNF71 KRAB expression (TPM ≥ 1.5) versus ZNF71 KRAB expression (TPM < 1.5) in patient tumors defined by four EMT phenotypes. The number of patient samples in each category is provided in the figure. (
) Distribution of ZNF71 KRAB expression (TPM ≥ 1.5) versus ZNF71 KRAB expression (TPM < 1.5) in patient tumors defined by four EMT phenotypes. The number of patient samples in each category is provided in the figure. (
E) Comparison of ZNF71 overall, KRAB-less, and KRAB expression in NSCLC cell lines categorized by median ZEB1 expression. (
) Comparison of ZNF71 overall, KRAB-less, and KRAB expression in NSCLC cell lines categorized by median ZEB1 expression. (
F) Fold change of ZNF71 overall, KRAB, and KRAB-less expression in Epithelial, Mesenchymal, and Mostly Mesenchymal NSCLC cell lines in qRT-PCR analysis (individual cell line results displayed in Figure 3A). * p-Value < 0.05, ** p-value < 0.01, and *** p-value < 0.001; NS (non-significance): p-value > 0.05 in ANOVA and Tukey’s tests. (
) Fold change of ZNF71 overall, KRAB, and KRAB-less expression in Epithelial, Mesenchymal, and Mostly Mesenchymal NSCLC cell lines in qRT-PCR analysis (individual cell line results displayed in Figure 3A). * p-Value < 0.05, ** p-value < 0.01, and *** p-value < 0.001; NS (non-significance): p-value > 0.05 in ANOVA and Tukey’s tests. (
G) Fold change of ZNF71 overall, KRAB, and KRAB-less expression in Epithelial and Mesenchymal/Mostly Mesenchymal (combined) NSCLC cell lines in qRT-PCR analysis. * p-Value < 0.05, ** p-value < 0.01, and *** p-value < 0.001; NS: p-value> 0.05 in two-sample t-tests. (
) Fold change of ZNF71 overall, KRAB, and KRAB-less expression in Epithelial and Mesenchymal/Mostly Mesenchymal (combined) NSCLC cell lines in qRT-PCR analysis. * p-Value < 0.05, ** p-value < 0.01, and *** p-value < 0.001; NS: p-value> 0.05 in two-sample t-tests. (
H,
and
I
) EMT classifiers in both patient tumors and cell lines were validated by stromal scores quantified with ESTIMATE [20]. (
H
) Comparison of stromal scores of patient tumors categorized by four EMT phenotypes. (
I) Comparison of stromal scores of NSCLC cell lines categorized by median ZEB1 expression.
) Comparison of stromal scores of NSCLC cell lines categorized by median ZEB1 expression.
The association between
ZNF71
and EMT in RNA-seq data was further analyzed with qRT-PCR and Western blots. The cell lines included in the qRT-PCR analysis (Figure 3A) can be categorized into four groups based on the protein expression of epithelial and mesenchymal markers in Western blots (Figure 3B): Epithelial (H441, H513, H820, and H358), Mesenchymal (H23, H460, and H1299), and Mostly Mesenchymal (A549 and H1395).
ZNF71 KRAB
and
KRAB
-less expression was significantly different among the three NSCLC cell line groups: Epithelial, Mesenchymal, and Mostly Mesenchymal (
p
< 0.05, ANOVA tests).
ZNF71 KRAB
expression was significantly higher in the Mesenchymal group than in the Epithelial group (
p
< 0.05, Tukey’s tests; Figure 4F).
ZNF71 KRAB
-less expression was significantly higher in the Mesenchymal group than in the Epithelial group and the Mostly Mesenchymal group (
p
< 0.05, Tukey’s tests; Figure 4F). When mesenchymal and mostly mesenchymal groups were combined in the analysis, only
ZNF71 KRAB
had a significantly higher expression in Mesenchymal/Mostly Mesenchymal group than in the Epithelial group (
p
< 0.05, two-sample
t
-tests; Figure 4G). Thus, the qRT-PCR and Western blot results further substantiated the association between
ZNF71 KRAB
and EMT observed in the RNA-seq data in NSCLC patient tumors and cell lines.
Stromal infiltration of the patient samples was assessed using the stromal scores computed with software ESTIMATE [20]. The patient tumors showing the highest average stromal score were in the Mesenchymal group, followed by High expression overlap, Low expression overlap, and Epithelial groups in descending order. The stromal scores of patient tumors defined as four EMT phenotypes were significantly different from each other (
p
< 0.001, ANOVA tests;
p
< 0.01, Tukey’s tests), except for the High-expression-overlap phenotype versus the Low-expression-overlap phenotype (Figure 4H). Since the NSCLC cell lines do not contain stromal cells, their average stromal scores are all negative (Figure 4I). Correlation of EMT markers and
ZNF71 isoforms with stromal and immune infiltration scores in patient tumors is provided in Table A4. These results validated our EMT classification of NSCLC tumors and cell lines.
isoforms with stromal and immune infiltration scores in patient tumors is provided in Table A4. These results validated our EMT classification of NSCLC tumors and cell lines.
4. Discussion
Lung cancer is difficult to manage in clinics due to its complex etiology and somatic mutations. There are currently no clinically available gene tests to predict metastasis and clinical benefits of chemotherapy for all NSCLC, stages 1 to 3A. The 7-gene assay has been validated in our previous study as prognostic and predictive of chemotherapeutic benefits in multiple U.S. hospitals and a clinical trial JBR.10. (
n
= 331) [6]. The 7-gene assay also estimates each individual patient’s response to four drugs used to treat lung cancer: cisplatin, carboplatin, paclitaxel, and pemetrexed [6]. Thus, the 7-gene assay could meet the critical need in clinics to identify specific NSCLC patients who are at risk for tumor recurrence/metastasis and would benefit from receiving adjuvant chemotherapy. In addition, this capacity would reduce the use of adjuvant therapy in circumstances in which no benefit, but potentially negative side effects, would result.
Many studies have investigated molecular alterations in single genes in early-stage NSCLC and their prognostic and predictive implications [29], such as mutations in K
Many studies have investigated molecular alterations in single genes in early-stage NSCLC and their prognostic and predictive implications [37], such as mutations in K
RAS
,
P53
,
EGFR [30],
[38],
STK11 [31], mRNA, and protein expression of ERCC1 [32][33]. Nevertheless, none of these genes are ready for primetime clinical applications as prognostic and predictive biomarkers for early-stage NSCLC [29]. Several prognostic/predictive gene expression signatures for early-stage NSCLC have been reported [34][35][36][37], among which the Razor 14-gene assay [36][38] (Razor Genomics, Brisbane, CA, USA) has been commercialized for the prognosis of early-stage non-squamous NSCLC and is currently recruiting patients for a clinical trial. However, the Razor 14-gene assay is not applicable for squamous cell lung carcinoma, which accounts for 26% of lung cancer cases [39]. The Myriad myPlan™ Lung Cancer and Pervenio™ Lung RS tests were commercially available in clinics for prognosis of early-stage NSCLC [40]. However, they are no longer listed in the Myriad All Products page [41]. FoundationOne CDx (Foundation Medicine, Inc, Cambridge, MA, USA) and Oncomine DX (Thermo Fisher Scientific Inc., Waltham, MA, USA) receive reimbursement coverage to match stage 4 NSCLC patients to specific proteasome inhibitor-based therapy based on their tumor genetic mutations. However, they do not predict metastasis for patients in stages 1 to 3A.
[39], mRNA, and protein expression of ERCC1 [40,41]. Nevertheless, none of these genes are ready for primetime clinical applications as prognostic and predictive biomarkers for early-stage NSCLC [37]. Several prognostic/predictive gene expression signatures for early-stage NSCLC have been reported [42,43,44,45], among which the Razor 14-gene assay [44,46] (Razor Genomics, Brisbane, CA, USA) has been commercialized for the prognosis of early-stage non-squamous NSCLC and is currently recruiting patients for a clinical trial. However, the Razor 14-gene assay is not applicable for squamous cell lung carcinoma, which accounts for 26% of lung cancer cases [47]. The Myriad myPlan™ Lung Cancer and Pervenio™ Lung RS tests were commercially available in clinics for prognosis of early-stage NSCLC [48]. However, they are no longer listed in the Myriad All Products page [49]. FoundationOne CDx (Foundation Medicine, Inc, Cambridge, MA, USA) and Oncomine DX (Thermo Fisher Scientific Inc., Waltham, MA, USA) receive reimbursement coverage to match stage 4 NSCLC patients to specific proteasome inhibitor-based therapy based on their tumor genetic mutations. However, they do not predict metastasis for patients in stages 1 to 3A.
Within the 7-gene signature, mRNA of
ZNF71
overall expression measured in qRT-PCR was positively correlated with survival in patients who received cisplatin, carboplatin, and Taxol, indicating that
ZNF71
mRNA is associated with chemosensitivity. Protein expression of ZNF71 was positively correlated with patient survival in independent tissue microarray cohort studies. However, mRNA of
ZNF71 overall expression measured in qRT-PCR was not found to be associated with NSCLC patient survival in the overall studied cohorts in our previous analysis [6]. Zinc finger proteins (ZFPs) are the largest family of transcription factors not only in human cells but also in most eukaryotes, representing about 3% of the total human genome [7][42]. A repeating motif within ZFPs contains two histidine and two cysteine amino acid residues (i.e., C2H2) coordinating zinc ion and has the ability to bind to DNA, RNA, or cellular proteins [8]. Therefore, ZFPs have a wide range of predicted functions according to their molecular structure, including DNA repair, degradation of proteins, signal transductions, migration of cells, regulation of apoptosis, lipid binding, and transcription regulation [7][8]. KRAB-ZFPs are a family of transcriptional repressors with diverse functions, most notably the silencing of transposable elements [43]. They contain an N-terminal KRAB domain and a C-terminal C2H2-type zinc finger array. The repressor action of KRAB-ZFPs requires the recruitment of KRAB-associated protein 1, also known as tripartite motif protein 28, KAP1/TRIM28. KAP1 functions as a scaffold complex composed of histone methyl transferase (SETDB1), heterochromatin protein-1 (HP-1), nucleosome remodeling and deacetylation (NuRD), and DNA methyl transferase [11]. When KRAB-ZFPs recruit KAP-1, the formed repressor complex leads to heterochromatin formation. Our group reported that KAP1 promotes proliferation and metastatic progression of breast cancer cells [44], consistent with the observed association between KAP1 and breast cancer progression [45]. RB-associated KRAB zinc finger (BRAK) was upregulated in NSCLC and was associated with poor prognosis in patients [44]. Zinc finger protein 668 (ZNF668) was reported to suppress NSCLC invasion and migration by down-regulating Snail and upregulating E-cadherin and zonula occludens-1 [46]. Down-regulated protein expression of ZNF668 was found in NSCLC tumors compared with normal lung tissues, and there was a negative association between ZNF668 protein expression and lymph node metastasis [46].
overall expression measured in qRT-PCR was not found to be associated with NSCLC patient survival in the overall studied cohorts in our previous analysis [6]. Zinc finger proteins (ZFPs) are the largest family of transcription factors not only in human cells but also in most eukaryotes, representing about 3% of the total human genome [7,50]. A repeating motif within ZFPs contains two histidine and two cysteine amino acid residues (i.e., C2H2) coordinating zinc ion and has the ability to bind to DNA, RNA, or cellular proteins [8]. Therefore, ZFPs have a wide range of predicted functions according to their molecular structure, including DNA repair, degradation of proteins, signal transductions, migration of cells, regulation of apoptosis, lipid binding, and transcription regulation [7,8]. KRAB-ZFPs are a family of transcriptional repressors with diverse functions, most notably the silencing of transposable elements [51]. They contain an N-terminal KRAB domain and a C-terminal C2H2-type zinc finger array. The repressor action of KRAB-ZFPs requires the recruitment of KRAB-associated protein 1, also known as tripartite motif protein 28, KAP1/TRIM28. KAP1 functions as a scaffold complex composed of histone methyl transferase (SETDB1), heterochromatin protein-1 (HP-1), nucleosome remodeling and deacetylation (NuRD), and DNA methyl transferase [11]. When KRAB-ZFPs recruit KAP-1, the formed repressor complex leads to heterochromatin formation. Our group reported that KAP1 promotes proliferation and metastatic progression of breast cancer cells [52], consistent with the observed association between KAP1 and breast cancer progression [53]. RB-associated KRAB zinc finger (BRAK) was upregulated in NSCLC and was associated with poor prognosis in patients [52]. Zinc finger protein 668 (ZNF668) was reported to suppress NSCLC invasion and migration by down-regulating Snail and upregulating E-cadherin and zonula occludens-1 [54]. Down-regulated protein expression of ZNF668 was found in NSCLC tumors compared with normal lung tissues, and there was a negative association between ZNF668 protein expression and lymph node metastasis [54].
In this study of public RNA-seq data,
ZNF71 KRAB
had a significantly higher expression than the
ZNF71 KRAB
-less isoform in NSCLC patient tumors [25] and cell lines [27]. The expression of both isoforms was significantly correlated in patient tumors and cell lines. Patients with higher
ZNF71 KRAB
expression had a significantly worse survival outcome than patients with lower
ZNF71 KRAB
expression, whereas the
ZNF71
overall expression and the
ZNF71 KRAB
-less isoform were not prognostic in the same patient cohort (
). In this study,
ZNF71
overall expression was not prognostic in RNA-seq data of NSCLC patient cohorts, which is consistent with our previous qRT-PCR results. We designed primers for
ZNF71 KRAB
and
KRAB
-less isoforms for TaqMan qRT-PCR assays, and the results in NSCLC cell lines confirmed the overall higher expression of
ZNF71 KRAB
than that of the
KRAB
-less isoform. Further investigation revealed an association between
ZNF71 KRAB
expression and EMT in RNA-seq data of NSCLC patient tumors and cell lines, which was validated in qRT-PCR and Western blot assays of cell lines (
and
).
ZNF71 KRAB
was overexpressed in cell lines resistant to docetaxel (
) and paclitaxel (
) treatment compared to chemo-sensitive cell lines, which was consistent with its association with poor prognosis in patients. Given the transcriptional repression role of the KRAB domain, the negative correlation between
ZNF71 KRAB
expression and NSCLC patient survival could be reasonable. These results indicate that the ZNF71 KRAB isoform is a more effective prognostic factor than
ZNF71
overall expression and the
ZNF71 KRAB
-less isoform for NSCLC. Further investigation is warranted to explore whether the
ZNF71 KRAB
isoform provides added prognostic and predictive value to the original 7-gene assay for NSCLC or whether
ZNF71 KRAB
could replace
ZNF71
overall expression in the 7-gene assay to achieve better prognostic performance.
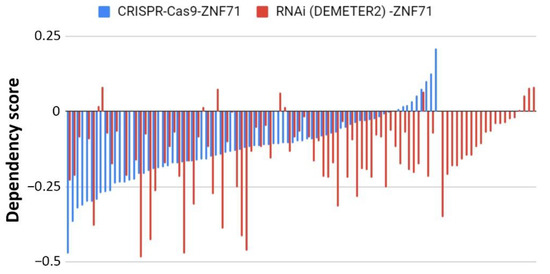
Figure 5. Functional analyses of ZNF71 in NSCLC cell lines using CRISPR-Cas9 and RNAi approaches. Dependency scores of ZNF71 in NSCLC cell lines using CRISPR-Cas9 (n = 78) and RNAi (normalized with DEMETER2; n = 92).
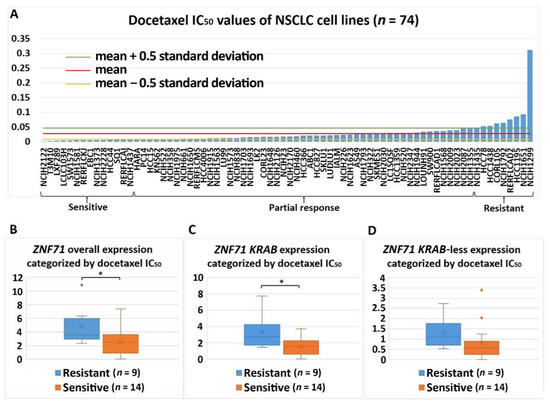
Figure 6. Association of the expression of
The expression of overall
ZNF71 and its isoform with docetaxel drug response (IC50) in NSCLC cell lines (n = 74). ZNF71 overall and KRAB expression was significantly higher in docetaxel-resistant cell lines compared with docetaxel-sensitive cell lines. (A) Docetaxel IC50 values of NSCLC cell lines. (B–D) Comparison of ZNF71 overall (B), KRAB-less isoform (C), and KRAB isoform (D) expression in cell lines categorized by IC50 values (resistant versus sensitive). * p < 0.05 in two-sample t-tests used in (B–D).
The expression of overall ZNF71 and its isoforms was not significantly different among different histological subtypes in patient tumors (
and its isoforms was not significantly different among different histological subtypes in patient tumors (
). The expression of
ZNF71
overall and the
KRAB
isoform was significantly different among histological subtypes in NSCLC cell lines (
). The observed discrepancy in
ZNF71
overall and
KRAB
expression among NSCLC histological subtypes in patient tumors and cell lines is possibly due to the fact that stromal cells are present in the NSCLC tumors but not in the studied cell lines. There was a strong association between
ZNF71
overall and
KRAB
expression and stromal infiltration in patient tumors (
) but not in NSCLC cell lines (results not shown). In the future, it would be interesting to investigate whether
ZNF71
overall and
KRAB
expression could differentiate NSCLC histological subtypes in micro-dissected epithelial cells from NSCLC tumors.
The role of EMT in cancer patient outcomes is not well defined and remains controversial [19][47]. In this study, we developed an EMT classifier based on transcriptional profiles of 14 EMT markers, and NSCLC patients defined with four EMT phenotypes had distinct survival outcomes (
The role of EMT in cancer patient outcomes is not well defined and remains controversial [19,55]. In this study, we developed an EMT classifier based on transcriptional profiles of 14 EMT markers, and NSCLC patients defined with four EMT phenotypes had distinct survival outcomes (
). Out of the 14 EMT markers, 13 (all except for
CTNNB1
) used in our classification were included in the pan-cancer EMT classifier presented in Panchy et al. [21], which also divides tumors into quadrants to characterize hybrid EMT states in tumors. Our EMT classifier was validated using stromal scores quantified with the well-established software ESTIMATE [20].
ZNF71 KRAB
expression was prognostic in NSCLC patients with high expression of EMT markers (the Epithelial group and the High expression overlap group) but not prognostic in tumors with low expression of EMT markers (the Mesenchymal group and the Low expression overlap group). These results imply that transcriptional biomarkers might have distinct prognostic implications in tumors with different EMT characteristics. The unclear role of EMT in patient outcomes is further complicated by tumor stromal and immune infiltration. For instance, the expression of
ZNF71
overall and its isoforms was not significantly different in NSCLC patient tumors separated by median
ZEB1
expression (the top 50%
ZEB1
expression group versus the bottom 50%
ZEB1
expression group, two-sample
t
-tests; results not shown). However, a strong association was found between
ZNF71 KRAB
and
ZEB1
gene expression in NSCLC epithelial cell lines (
E).
ZNF71 molecular functions have not been reported in the literature. This study evaluated the functional associations of ZNF71 using publicly available genome-scale CRISPR-Cas9 and RNAi screening data. The knockouts and knockdowns in NSCLC cell lines were performed for overall ZNF71, i.e., all transcripts. They did not affect cell proliferation (
). Concordantly, overall
ZNF71
mRNA expression was not associated with patient survival. We found that
ZNF71 KRAB
was associated with patient outcome and with EMT, i.e., expressed higher in the top 50
ZEB1 expressing cells. A mechanistic link between ZNF71 KRAB and EMT is currently not known. We could speculate that EMT is associated with significant changes in splicing and hence can potentially skew splicing for inclusion of the KRAB domain [48][49]. The link between other KRAB-ZFPs and EMT was reported. TRIM28 protein, a universal co-factor for KRAB-ZFP transcription factors [22], is known to participate in a wide range of aspects of cellular biology, either promoting cell proliferation [44] or mediating anti-proliferative activities [50]. TRIM28 protein is involved in cancer by regulating gene expression through heterochromatin formation, mediation of DNA damage response, inhibition of p53 activity, regulation of EMT, and maintenance of stem cell pluripotency and genome stability [51]. TRIM28 expression is induced following transforming growth factor-β (TGF-β) treatment at both protein and mRNA levels. TRIM28 deficiency impairs TGF-β-induced EMT and decreases cell migration and invasion, and the expression of TRIM28 affects the acetylation of histones on E-cadherin and N-cadherin promoters, suggesting that TRIM28 contributes to EMT and might be important for tumor metastasis in lung cancer [23]. ZNF382 KRAB regulates EMT and functions as a tumor suppressor in gastric cancer [24]. To gain better insight into the mechanistic link between ZNF71 KRAB and EMT, we are currently conducting genome-scale network analysis to identify all the genes showing a significant statistical association with
expressing cells. A mechanistic link between ZNF71 KRAB and EMT is currently not known. We could speculate that EMT is associated with significant changes in splicing and hence can potentially skew splicing for inclusion of the KRAB domain [56,57]. The link between other KRAB-ZFPs and EMT was reported. TRIM28 protein, a universal co-factor for KRAB-ZFP transcription factors [22], is known to participate in a wide range of aspects of cellular biology, either promoting cell proliferation [52] or mediating anti-proliferative activities [58]. TRIM28 protein is involved in cancer by regulating gene expression through heterochromatin formation, mediation of DNA damage response, inhibition of p53 activity, regulation of EMT, and maintenance of stem cell pluripotency and genome stability [59]. TRIM28 expression is induced following transforming growth factor-β (TGF-β) treatment at both protein and mRNA levels. TRIM28 deficiency impairs TGF-β-induced EMT and decreases cell migration and invasion, and the expression of TRIM28 affects the acetylation of histones on E-cadherin and N-cadherin promoters, suggesting that TRIM28 contributes to EMT and might be important for tumor metastasis in lung cancer [23]. ZNF382 KRAB regulates EMT and functions as a tumor suppressor in gastric cancer [24]. To gain better insight into the mechanistic link between ZNF71 KRAB and EMT, we are currently conducting genome-scale network analysis to identify all the genes showing a significant statistical association with
ZNF71
at gene expression and DNA copy number variation levels in NSCLC patient tumors. We have identified ZNF71-mediated molecular association networks in EMT using public data. A similar analysis will be conducted to identify ZNF71 KRAB mediated molecular networks in EMT using public RNA-seq data generated from NSCLC patient tumors. In the future knockdown/overexpression experiments, we will examine which of these EMT-relevant genes in the identified networks are affected by ZNF71 KRAB to investigate the potential mechanistic link between ZNF71 KRAB and EMT in NSCLC.
The association between ZNF71 and its isoforms in terms of chemoresponse is also complex due to different genetic predisposition to chemotherapeutic regimens in tumor cells. In the studied NSCLC cell line panel,
ZNF71 KRAB
overexpression was observed in tumor cells that were resistant to Taxol compared to Taxol-sensitive tumor cells (
Figure 7). These results were consistent with the observed negative association of
9). These results were consistent with the observed negative association of
ZNF71 KRAB
expression and patient survival (
). In the H460 cell line that is defined as having a partial response to Taxol in the studied cell line panel (
Figure 7A),
9A),
ZNF71
overall expression was significantly lower in H460-R than in H460-P cells (
Figure 8). These results were consistent with the observed association of
10). These results were consistent with the observed association of
ZNF71
overall expression with chemosensitivity to Taxol in NSCLC patients [6]. Significantly higher
ZNF71
overall and
KRAB expression in docetaxel-resistant cell lines suggests their potential use in predicting predisposition to this agent or in combined immunotherapy and chemotherapy, based on the recent report of the efficacy of the combination of docetaxel and pembrolizumab in treating NSCLC [52].
expression in docetaxel-resistant cell lines suggests their potential use in predicting predisposition to this agent or in combined immunotherapy and chemotherapy, based on the recent report of the efficacy of the combination of docetaxel and pembrolizumab in treating NSCLC [33].
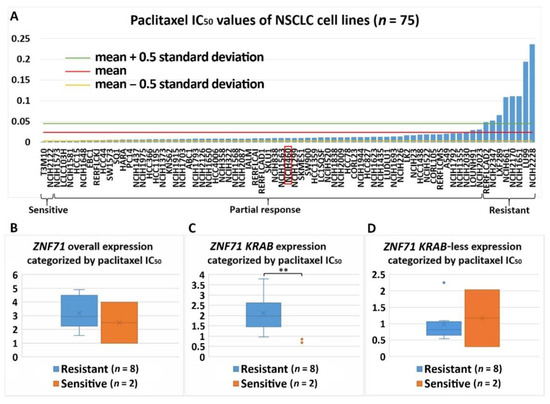
Figure 7. Association of the expression ZNF71 and its isoform with paclitaxel drug response (IC50) in NSCLC cell lines (n = 75). ZNF71
overall and
KRAB expression was significantly higher in paclitaxel-resistant cell lines compared with paclitaxel-sensitive cell lines. (A) Paclitaxel IC50 values of NSCLC cell lines. (B–D) Comparison of ZNF71 overall (B), KRAB-less isoform (C), and KRAB isoform (D) expression in cell lines categorized by IC50 values (resistant versus sensitive). ** p < 0.01 in two-sample t-tests used in (B–D).
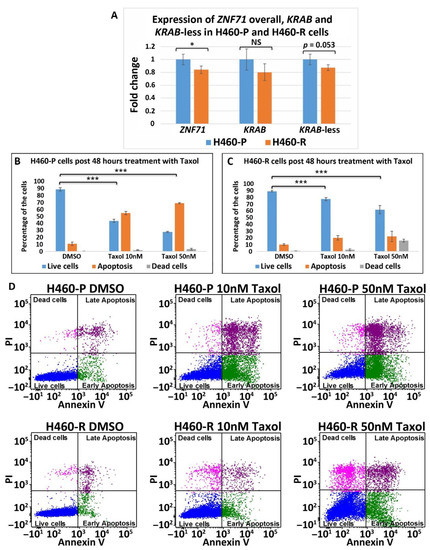
Figure 8. ZNF71 isoforms in paclitaxel-resistant (H460-R) and parental (H460-P) cell lines. (A) Comparison of fold change of ZNF71 overall, KRAB, and KRAB-less expression in H460-P and H460-R cell lines in qRT-PCR experiments. * p < 0.05, *** p < 0.001, NS: non-significance, two-sample t-tests. (B,C) H460-P (B) and H460-R (C) cells were treated either with DMSO or Taxol with the indicated doses. After 48 h, the cells were stained with Annexin V and PI and the percent of live/dead cells was determined by flow cytometry. Error bars represent standard deviation from three biological replicates. A mixed-effect model was used to assess the difference between two conditions, using R package Ime4. The p-value was calculated based on asymptotic Z-distribution. (D) Flow cytometry images for H460-P and H460-R, respectively, after treatment with either DMSO or Taxol.
ZNF71 overall and KRAB expression was significantly higher in a normal lung small airway epithelial cell line (SAEC) compared with that in most NSCLC cell lines analyzed in qRT-PCR. Since we cannot draw any solid conclusion based on one normal lung cell line, we did not show these results. One possible explanation could be that SAEC cells were cultured in specialized media (Lonza, Basel, Switzerland) with added defined growth factors, while the lung cancer cell lines were cultured in standard DMEM plus 10% FBS. Many KRAB-ZFPs were reported to act as either tumor suppressors or oncogenes [53]. ZNF382 is down-regulated in multiple carcinoma types due to promoter methylation and functions as a tumor suppressor in gastric cancer [24]. ZNF23, a KRAB-containing protein, is down-regulated in human cancers and inhibits cell cycle progression [54]. RB-associated KRAB (RBAK) zinc finger is upregulated in NSCLC and promotes cell migration and invasion [55]. We are planning to carry out the following analysis of ZNF71 overall and isoform expression in our future study: (1) examine multiple normal lung epithelial cell lines in qRT-PCR, (2) analyze TCGA data for NSCLC tumors versus normal lung tissue samples, and (3) design knockdown and overexpression of ZNF71 in vitro and/or in vivo xenograft studies to examine whether it is oncogenic or tumor suppressive.
expression was significantly higher in a normal lung small airway epithelial cell line (SAEC) compared with that in most NSCLC cell lines analyzed in qRT-PCR. Since we cannot draw any solid conclusion based on one normal lung cell line, we did not show these results. One possible explanation could be that SAEC cells were cultured in specialized media (Lonza, Basel, Switzerland) with added defined growth factors, while the lung cancer cell lines were cultured in standard DMEM plus 10% FBS. Many KRAB-ZFPs were reported to act as either tumor suppressors or oncogenes [60]. ZNF382 is down-regulated in multiple carcinoma types due to promoter methylation and functions as a tumor suppressor in gastric cancer [24]. ZNF23, a KRAB-containing protein, is down-regulated in human cancers and inhibits cell cycle progression [61]. RB-associated KRAB (RBAK) zinc finger is upregulated in NSCLC and promotes cell migration and invasion [62]. We are planning to carry out the following analysis of ZNF71 overall and isoform expression in our future study: (1) examine multiple normal lung epithelial cell lines in qRT-PCR, (2) analyze TCGA data for NSCLC tumors versus normal lung tissue samples, and (3) design knockdown and overexpression of ZNF71 in vitro and/or in vivo xenograft studies to examine whether it is oncogenic or tumor suppressive.
