Atmospheric plasma treatment is an effective and economical surface treatment technique. The main advantage of this technique is that the bulk properties of the material remain unchanged while the surface properties and biocompatibility are enhanced.
- plasma
- atmospheric pressure
- cell cultivation
- polymer
- surface modification
1. Introduction
In the past decades, scientific advances in tissue engineering have created a unique opportunity to fabricate or improve biological substances that replace, repair, maintain, or enhance tissue functions. Biomaterial engineering is an essential aspect of the tissue engineering field that relies on a combination of materials, cells, and bioactive molecules; to cause a desirable cell-biomaterial interaction within the host environment [1,2][1][2]. Polymers have been widely utilized in biomedical applications due to their bulk properties including physical, chemical, and biological properties. In addition to bulk properties, specific surface properties, such as surface free energy, hydrophilicity, and surface morphology, are required in particular medical applications [3,4][3][4]. However, polymers do not possess the desirable surface properties required for these applications [5].
In cell-material interaction, the initial and most essential event is protein adsorption. The adsorbed protein layer composition and structure depend on the material surface chemical and physical properties; and determine the cellular response and adhesion strength as illustrated in Figure 1. The adhesion of cells is initiated by the adhesion of integrins, which are a receptor protein that is located on cell membrane, with adsorbed protein layer. Following this process, the spreading of cells and enhancing their surface contact area are initiated due to the formation of stress fibers, which are filaments of actin. Finally, strong points of attachment “Focal adhesion” are formed, and the cells are strongly attached to the biomaterial surface. In order to induce cell adhesion, a moderately wettable material surface is preferred [6,7,8][6][7][8]. However, polymer’s surfaces are mostly hydrophobic with low surface energy. In this regard, surface modification of polymers is needed to form a cell-biomaterial bonding surface.
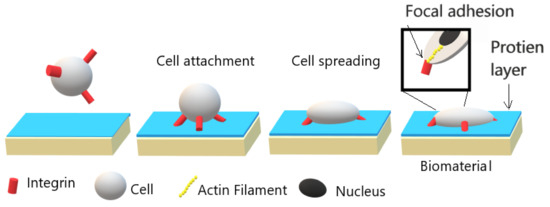
Figure 1. Illustration of cell-biomaterial interaction and its relationship with the strength of adhesion. Adapted with permission from ref. [6]. Copyright 2005 Elsevier.
Several methods for surface modification to enhance cell-polymer interaction have been reported in the literature; including physio-chemical, mechanical, and biological treatments [9]. For instance, Suggs [10] used Kr-F excimer laser, which is one of the physio-chemical surface treatments, to modify polymethylmethacrylate (PMMA), polyethylene (PE), terephthalate (PETG), and polytetrafluoroethylene (PTEF) surfaces. The observation was that a high level of UV radiation was sufficient to cause surface damage on PMMA and PTEF; while low radiation dose increased surface roughness of treated polymers, which resulted in increased cell adhesion on PMMA, PETG, and PTEF surfaces. Moreover, UV surface modification was investigated by Olbrich and coworkers [11]. A nanocomposite surface for human umbilical vein endothelial cell (HUVEC) cultivation was modified. It was shown that the formation of N and O functional groups have increased cell growth significantly. Svorcik et al. [12] grafted carbon nanoparticles (CNPs) onto a polyethylene terephthalate (PET) and high-density polyethylene (HDPE) surfaces previously treated with argon plasma. Adhesion and proliferation of vascular smooth muscle cells (VSMC) showed a positive effect for surface CNPs grafting.
Any type of surface modification techniques will affect surface chemistry and morphology, which will modify the substrate mechanical, optical, adhesive, electrical, and morphological properties. These changes should occur in small depth, while the bulk properties remain unaltered [13]. During the last years, atmospheric pressure plasma treatment has been confirmed to be an effective technique for modification of polymer’s surface, especially polymers that are utilized in biomedical applications. The main goal of atmospheric plasma treatment generally is the formation of functional groups on the surface of the polymer. Consequently, polymer’s surface properties including wettability, biocompatibility, and surface chemistry are enhanced. In this technique, only surface properties are changed, the bulk properties of the polymers remain unaffected. Furthermore, it is a solvent free technique that has the ability to modify complex-shaped surfaces. Several methods have been reported in the literature to generate atmospheric pressure plasma according to excitation energy including corona discharge [14], dielectric barrier discharge [15], and atmospheric pressure plasma jet [16]. Several research groups investigated the atmospheric pressure plasma surface modification of polymers and its influence on cell cultivation. For instance, Poly (l-lactic acid) surface modification with corona discharge has been evaluated by Dolci et al. [17]. Fibroblast cells ware cultivated on plasma treated surface, in order to evaluate the surface treatment technique. The results showed that the fibroblasts morphology that adhered on the treated surface was enhanced. Moreover, dielectric barrier discharge plasma treatment of PCL scaffold surface was investigated by Yildrem et al. [18]. The cultivation of osteoblasts on plasma treated surface enhanced the initial attachment, proliferation, and migration of cells.
In this review, we focus on atmospheric pressure plasma treatment of polymeric materials surface and its effect on different cell line cultivation. In the beginning, a brief overview of plasma generation mechanism and the difference between thermal and non-thermal plasma have been mentioned. Furthermore, atmospheric pressure plasma classifications have been discussed in detail, in addition to the plasma gas active species. The second section in this review, describes different modifications of atmospheric pressure plasma treatment on polymer surface including removal of contamination, etching, introducing functional groups, and grafting. The following section demonstrates the cell-biomaterial interaction mechanism and its relationship with biomaterial surface physical and chemical properties. In this section, several studies regarding the interaction of different types of cell lines, including fibroblast, osteoblast, osteosarcoma, endothelial cells, cardiomyocytes, and mesenchymal stem cells with atmospheric pressure plasma treated polymers, have been discussed and summarized. Finally, the challenges and future aspects from our point of view, in addition to the conclusion are mentioned.
2. Atmospheric Pressure Plasma Technology
Plasma is usually referred to as the fourth state of matter. The “plasma” concept was first introduced by the scientist Irving Langmuir in 1928. It consists of atoms, molecules, ions, which are atoms with some removed orbital electron, electrons, and radicals. Plasma can be artificially generated by applying energy to a gas in order to produce ionized gaseous substances containing positive ions and negative electrons. Therefore, from a macroscopic point of view plasma is electrically neutral, even though it contains free charge carrier and it is electrically conductive, so generally plasma exhibits “quasi-neutral” Behavior [19]. The energy that is used to generate plasma can be thermal energy or carried out by applying electrical field, magnetic field or even nuclear reaction [20].
2.1. Plasma Generation
The atmospheric pressure plasma, described in this review, is generated by applying electrical field to a neutral gas. Either direct current excitation or alternating current excitation, at a frequency range that lies from low frequencies to several GHz, are applied to a gas at atmospheric pressure. The selected plasma gas or gases mixture is generally performed between two plates for plasma generation. Three major reactions that take place in plasma generation are excitation, ionization, and dissociation. Any volume of neutral gas contains few charge carriers, which are electrons and ions. After applying the electrical field to the plasma gas these free charge carriers are accelerated and may collide with atoms in the gas or with the surface of the electrodes. Therefore, the excitation process increases the translational energy of the gas atoms, and transitions internal energy to higher state. If sufficient energy is applied in excitation process, the most loosely bound electrons are removed from the atom, which is ionization. Both excitation and ionization may be due to the reaction by electron collision, ion collision, neutral particle collision, and radiation. On the other hand, dissociation is a result of an inelastic collision of a molecule with an electron, ion, or photon [21]. Generally, a vacuum pump is applied to the plasma generator chamber in order to generate plasma, but in atmospheric pressure plasma generation no need for vacuumed chamber. Figure 2 shows the general plasma generator scheme.
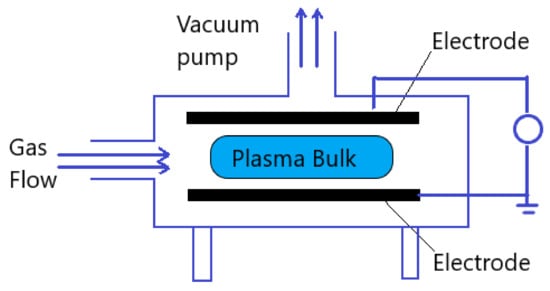
Figure 2. Schematic diagram of general plasma generator.
The plasma can be classified into two groups according to the temperature of electrons or thermodynamic equilibrium; which are:
-
Local thermodynamic equilibrium (LTE) (or thermal) plasma, and
-
Non-local thermodynamic equilibrium (non-LET) (or non-thermal) plasma.
-
Local thermodynamic equilibrium (LTE) (or thermal) plasma, and
-
Non-local thermodynamic equilibrium (non-LET) (or non-thermal) plasma.
The LET or thermal plasma is composed of electrons, ions, and neutrals in thermal equilibrium with each other, and background gas is heated up to a few thousands of Kelvin degrees. On the other hand, the non-LET or non-thermal plasma temperature of electrons, ions, and neutrals are quite different, generally, the electron temperature is at a much higher temperature than other particles. The background gas temperature in non-thermal plasma is quite low. Therefore, the discharge energy mostly goes into the production of energetic electrons. Owing to the low temperature of non-thermal plasma, it is capable to treat thermolabile materials with this plasma. Moreover, non-thermal plasma is easily adapted to complex geometric. Therefore, it is widely used in material surface modification, medical sterilization, and microbial decontamination applications [22].
Plasma is generated under different gas pressure. In the past several decades, atmospheric pressure plasma has been explored to avoid the expensive and complicated vacuum system. As described above, atmospheric pressure plasma can be classified as thermal and non-thermal plasma [23]. Mostly, in polymeric material surface treatment application non-thermal plasma has been utilized, also it is generated by applying electrical field at atmospheric pressure.
2.2. Classification
Atmospheric pressure plasma is an economical and easy-to-use technology utilized in many applications including surface modification. Several methods have been reported in the literature to generate plasma at atmospheric pressure. In general, these methods can be classified according to excitation frequency as follows:
-
DC and low-frequency discharges such as corona discharges and dielectric barrier discharges,
-
Radiofrequency discharges such as atmospheric pressure plasma jets, and
-
Microwave induced plasmas.
-
DC and low-frequency discharges such as corona discharges and dielectric barrier discharges,
-
Radiofrequency discharges such as atmospheric pressure plasma jets, and
-
Microwave induced plasmas.
The DC and low-frequency discharge can work either with a continuous or a pulsed mode depending on their design. The continuous working mode; for example, the arc plasma torches, are mostly thermal plasma. On the other hand, the pulsed mode; such as corona and dielectric barrier discharges (DBD), are generally non-thermal treatment which is suitable for polymer surface treatment.
The radiofrequency discharge is generated by applying a radiofrequency electrical field on a flow of gas or a mixture of gases. Regarding their structure, the radio frequency discharge can be classified as capacitive or inductive coupled. The capacitively coupled plasma discharge (CCP) is a low power discharge; in contrast, the inductively coupled plasma (ICP) is a high-powered discharge working in the kW range [24].
The microwave induced plasmas (MIPs) are electrode-less systems. All the MIPs work under the same principle that is microwaves guided along the system, and the energy is transmitted to plasma gas. Several elastic collisions between plasma gas electrons and heavy particles occur. Therefore, the electron gets enough energy to produce inelastic collisions and the gas is partially ionized and becomes plasma. All the MIPs consist of:
-
A microwave power source;
-
microwave equipment for example waveguide;
-
an ignition system; and
-
gas injection system. As shown in Figure 3.
-
A microwave power source;
-
microwave equipment for example waveguide;
-
an ignition system; and
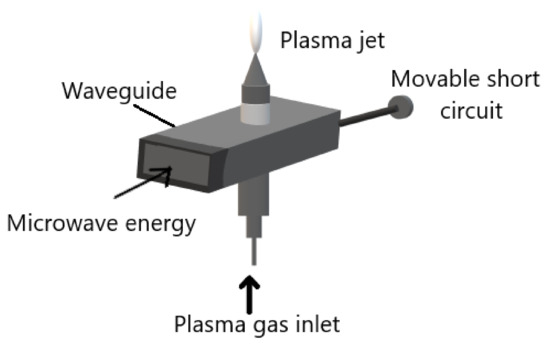
Figure 3. Schematic diagram of microwave induced plasma system. Adapted with permission from ref. [26][25]. Copyright 1984 IOP Publishing LTD.
The microwaves are generated using a magnetron at a frequency of a few GHz. It is guided to the system by a waveguide or coaxial cable. The gas temperature of the plasma can vary from room temperature to few thousand degrees. Microwave plasma torch is an example of MIPs which creates plasma that flows in the air. The electrodes-free system allows it to be used as a torch which is easily used and applied. Furthermore, the ease of ignition utilizing any gas or gas mixture is also another advantage of the MIP system [25][26].
Although different atmospheric pressure plasma sources have been studied through the years for surface modification applications, the vast majority of studies are dealing with corona discharges, dielectric barrier discharges, and atmospheric pressure plasma jet. Therefore, here we discuss these plasma sources in detail, mentioning their basic configurations, applications, advantages, and disadvantage.
2.2.1. Corona Discharges
Corona discharge generates plasma on a sharp point, edge, or thin wire, under atmospheric pressure with mostly air as a plasma gas. The corona discharge is spatially non-uniform, and the ionization and the electrical field mostly are located near the pin-shaped electrode. The most popular configuration of corona discharge is the point-to-plane, whereas the strong electrical field generated between the sharp point and the flat electrode results in ionization of the gas plasma. Figure 4 demonstrates the point-to-plane corona discharge general configuration. However, the most popular corona discharge configurations in industrial applications are the wire-to-cylinder, wire-to-plane, and saw-to-blade [27]. The reasons for that are the homogeneous distribution of plasma discharges, and the ease of implementation. The sharp electrode in corona discharges is either positive or negative and accordingly the discharge is called positive or negative corona, respectively [28].
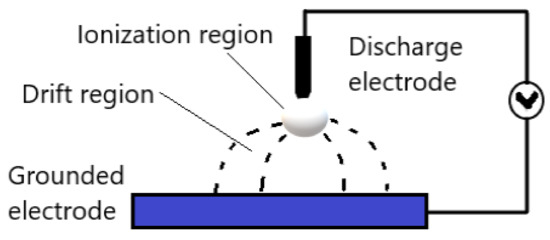
Figure 4. Schematic diagram of general point-to-plane corona discharge. Adapted with permission from ref. [36][29]. Copyright 1969 Elsevier (Open Access).
Although corona discharge is a versatile and environmentally friendly technique for surface treatment and ozone generation, it is still not gained much commercial momentum due to some disadvantages with such discharge such as non-uniformity and power loss. Furthermore, corona discharge volume is very small and due to that, the treatment surface is very small [29][30]. Because of the corona discharge advantages, including the ease of implementation, it has numerous applications for example, removal of unwanted volatile organics from the atmosphere [30][31], material’s surface treatment [31][32], gaseous contamination treatment [32][33], water purification [33][34], and ozone production [34][35]. Furthermore, the negative corona discharge have been studied for the possible use for treatment of microscopic cavities in dentistry due to its antibacterial effect and hairline geometry [35][36].
2.2.2. Dielectric Barrier Discharges
The dielectric barrier discharge (DBD) is plasma established in a gas gap between different electrodes configurations, with an insulating material between the electrodes. Typically, the insulating materials “barrier” such as glass, quartz, ceramics, plastics, and silicon rubber are used. The classical configurations of DBDs are either planar or cylindrical with at least one of the electrodes is covered with dielectric layer, typical electrodes arrangements are shown in Figure 5. Furthermore, several novel configurations of DBDs have been developed in order to adapt with many different applications, including sliding discharge, capillary plasma electrode discharge, microcavity plasma array, and coplanar configurations [37]. Plasma in DBDs is generated by applying high voltages; in the range of some kV. In order to achieve stable performance, the alternating current frequency range from low frequencies up to the order of several MHz. The gap between the electrodes is usually filled with gas or a mixture of gases and can vary from a few micrometers to some centimeters [37,38,39,40][37][38][39][40]. DBD usually exhibits two different modes depending on electrode geometries and setups; which are Filamentary and diffuse discharges. The filamentary mode is non-uniform discharges with many small discharge channels along the electrode called microdischarges. However, the filamentary DBD appears to be uniform to the naked eye or slow cameras after a long duration treatment or if the number density of microdischarges per cycle is high [41,42,43][41][42][43]. On the other hand, DBD diffuse mode is a uniform and homogeneous discharge [39]. Generally, the filamentary mode are utilized in DBD applications, because the diffuse mode operates under special conditions [44,45][44][45].
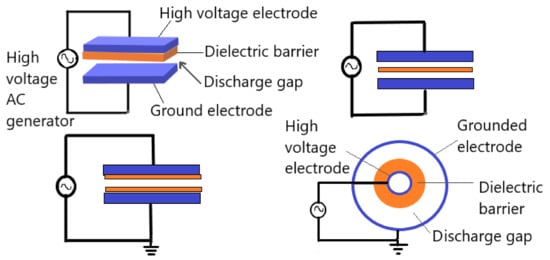
Figure 5. General planer and cylindrical dielectric barrier discharge geometrical configurations. Adapted with permission from ref. [45]. Copyright 2003 Elsevier.
The DBDs have many advantages, including low energy consumption contrary to corona discharges, low operational cost, scalability, effectiveness, and short processing time. Whereas, the main drawbacks are the high ignition voltage, and limited discharge gap height that is directly related to plasma homogeneity [46]. Many applications of DBD plasma, according to its advantages, are available such as ozone generation, excitation of CO2 lasers and excimer lamp, wastewater purification, plasma chemical vapor deposition, and surface modifications. Furthermore, DBD plasma has a huge antimicrobial potential, therefore it is used to improve food safety and extend the food shelf life [47]. Moreover, DBD has been widely used in medical applications including cancer treatment, dentistry, and regenerative medicine [48].
2.2.3. Atmospheric Pressure Plasma Jets
The atmospheric plasma jet (APPJ) is a plasma that is generated in non-sealed electrode arrangement and projected outside the electrode arrangement into the environment. The unique feature of APPJ resides in the fact that the plasma is generated within the device and extends in the open space in the form of “plasma plume”. Many different APPJ devices have been developed so far, differing in the electrode’s arrangement, strategy exploited for plasma generation, and shape and dimension of plasma plume. Generally, almost all the plasma jets generated within a duct through which the plasma gas or gases mixture flows and propagates. Furthermore, the plasma propagates through the duct and ejected outside the device, along the gas flow direction. The APPJ can be classified according to the electrode configuration and power supplies into corona plasma jet, DBD-based plasma jet, radiofrequency (RF)-generated plasma jet, and microwave (MW)-driven plasma jet.
The RF-generated plasma jet can be capacitively driven or inductively driven, and usually operate in the frequency range 1–100 MHz. The RF-generated plasma jet general capacitive configuration is reported in Figure 6a. The RF-generated plasma jet operates in RF power, which generates a very uniform and stable discharge between two concentric metallic electrodes.
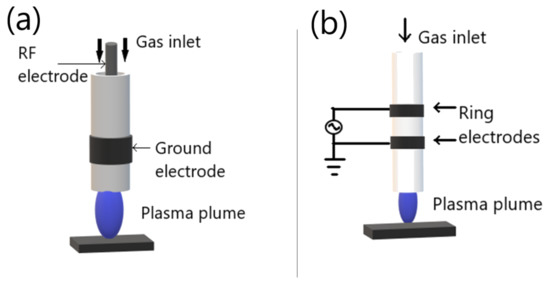
Figure 6. Schematic diagram of general APPJ: (a) RF-generated plasma jet; (b) DBD-based plasma jet. Adapted with permission from ref. [51][49]. Copyright 2017 Elsevier.
On the other hand, the general DBD-based plasma jet is shown in Figure 6b, two ring electrodes are coaxially arranged around a dielectric tube. The application of gas flow is parallel to the electrical field generated in the ring electrodes. Different parameters control the propagation of plasma plume from the device exist, including the electrical field geometry, the gas flow conditions, and the source design [49,50,51][50][51][49].
In addition to the advantages of the atmospheric pressure plasma including manageability and cost-effectiveness, the APPJ small plasma dimensions, penetrability, and distinctive remote operation, give the ability for plasma jet to treat materials with complex geometries and micro-structured pores. Nevertheless, the main drawback of APPJ is the small spot size which makes it inappropriate for homogeneous treatment of large surfaces [52]. The APPJ has received a significant attention, due to its advantages, in various fields of science and technology. For instance, deposition of coating, generation of nanoparticles, surface modification, and biomedical applications are some of APPJ applications [53]. As an example, Bernhardt and coauthors [54] have showed that different APPJs could be a promising and inexpensive treatments for many diseases, including atopic eczema, itch, pain relief, and epidermal barrier defects. Whereas APPJ already reached standard medical care status for wound healing and scar treatment.
2.3. Active Species
Plasma treatment is a widely used technique to modify polymeric surfaces. Argon (Ar), helium (He), nitrogen (N2), and oxygen (O2) are mainly the gases used in plasma generation for polymeric surface modification. The interaction between the active species in plasma generation using these gases and the polymer’s surface leads to the formation of free radicals at the surface of polymers [55]. For instance, using oxygen as plasma gas leads to a functional group containing-oxygen atom, for example, hydroxyl group, carbonyl group, and in some cases carboxylic group. A different functional group can be introduced into the surface of polymers depends on the gas or gases mixture used in plasma generation [7]. A list of plasma gases and their applications is listed in Table 1.
Table 1. A representative overview of atmospheric pressure plasma treatment of polymers and influence on fibroblast cell cultivation.
| Substrate | Plasma Type | Plasma Gas | Grafted or Deposited Layer | Cell Type | Observation | Reference |
|---|---|---|---|---|---|---|
| PCL | APPJ | Ar | PCL homopolymer PCL/PEG copolymer |
L929 | Enhancement in cell adhesion, proliferation and growth for deposited PCL/PEG scaffold | [94][56] |
| PCL/Chitosan/PCL | DBD | Ar/O2 Ar/N2 |
- | L929 | Increased cell viability, attachment and proliferation for Ar/O2 treatment. | [95][57] |
| PCL/Chitosan/PCL | DBD Plasma jet |
Ar/O2 Ar/N2 Dry air |
- | MRC5 | Enhancement of initial cell attachment and seven-day cell viability, proliferation, and growth for both treatments in comparison with the untreated scaffold. | [96][58] |
| LDPE | DBD | Ar /O2 | - | NIH 3T3 | Improvement in adhesion and cytocompatibility of cells. | [98][59] |
| PLLA | Linear corona discharge | N2 | - | MEF | Higher field/body area ratio Reduced percentage of apoptotic nuclei elongated with dendritic morphology |
[17] |
| PTFE and POx copolymer | DCSBD | Ar/air | - | 3T3 | Increased cells adhesion number | [102][60] |
| PS | APPJ | Air | - | L929 | Increase in the number of the attached fibroblast Wider spreading phenotype Vinculin protein and PTK2 increased |
[103][61] |
Generally, plasma gases are classified into five different types, which are:
-
Oxidizing gases such as O
2, air, N
2O, and H
2O;
-
Reducing gases such as H
- 2;
-
Nitrogen containing gases such as NH
- 3
,
-
Fluorine containing gases such as CF
4and SF
6, and
-
Polymerizing gases which use monomer gases to direct polymerize or graft a layer onto a substrate.
Each type of plasma gases has different application, especially in surface modification. For instance, oxidizing gases are utilized in leaving oxygen species on polymer surface or removal of organic contamination by oxidation, while reducing gases provide replacement of F or O in surface. On the other hand, nitrogen containing gases are used to generate amino group on substrate surface, which improve biocompatibility, wettability, and bondability of substrate. Whereas fluorine containing gases provide surface etching and plasma polymerization which make surface inert and hydrophobic [56,57][62][63].
(References would be added automatically after the entry is online)
References
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for Tissue Engineering. Ann. Biomed. Eng. 2014, 42, 323–337.
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue Engineering: Strategies, Stem Cells and Scaffolds. J. Anat. 2008, 213, 66–72.
- Pinson, J.; Thiry, D. Surface Modification of Polymers: Methods and Applications; John Wiley & Sons: Weinheim, Germany, 2020; ISBN 978-3-527-34541-0.
- Surucu, S.; Turkoglu Sasmazel, H. Development of Core-Shell Coaxially Electrospun Composite PCL/Chitosan Scaffolds. Int. J. Biol. Macromol. 2016, 92, 321–328.
- Primc, G. Recent Advances in Surface Activation of Polytetrafluoroethylene (PTFE) by Gaseous Plasma Treatments. Polymers 2020, 12, 2295.
- García, A.J. Get a Grip: Integrins in Cell–Biomaterial Interactions. Biomaterials 2005, 26, 7525–7529.
- Ferreira, P.; Alves, P.; Coimbra, P.; Gil, M.H. Improving Polymeric Surfaces for Biomedical Applications: A Review. J. Coat. Technol. Res. 2015, 12, 463–475.
- Horbett, T.A.; Latour, R.A. 2.1.2—Adsorbed Proteins on Biomaterials. In Biomaterials Science, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Massachusetts, MA, USA, 2020; pp. 645–660. ISBN 978-0-12-816137-1.
- Jaganathan, S.K.; Balaji, A.; Vellayappan, M.V.; Subramanian, A.P.; John, A.A.; Asokan, M.K.; Supriyanto, E. Review: Radiation-Induced Surface Modification of Polymers for Biomaterial Application. J. Mater. Sci. 2015, 50, 2007–2018.
- Suggs, A.E. Kr-F Laser Surface Treatment of Poly(Methyl Methacrylate), Glycol-Modified Poly(Ethylene Terephthalate), and Polytetrafluoroethylene for Enhanced Adhesion of Escherichia Coli K-12. Master’s Thesis, Virginia Tech, Blacksburg, WV, USA, 2002.
- Olbrich, M.; Punshon, G.; Frischauf, I.; Salacinski, H.J.; Rebollar, E.; Romanin, C.; Seifalian, A.M.; Heitz, J. UV Surface Modification of a New Nanocomposite Polymer to Improve Cytocompatibility. J. Biomater. Sci. Polym. Ed. 2007, 18, 453–468.
- Švorčík, V.; Makajová, Z.; Slepičková Kasálková, N.; Kolská, Z.; Žáková, P.; Karpíšková, J.; Stibor, I.; Slepička, P. Cytocompatibility of Polymers Grafted by Activated Carbon Nano-Particles. Carbon 2014, 69, 361–371.
- Sharma, R.; Sims, R.A.; Mazumder, M.K. Modification of Surface Properties of Polymeric Materials. J. Ark. Acad. Sci. 2002, 56, 6.
- Xu, W.; Liu, X. Surface Modification of Polyester Fabric by Corona Discharge Irradiation. Eur. Polym. J. 2003, 39, 199–202.
- Liu, C.; Cui, N.; Brown, N.M.D.; Meenan, B.J. Effects of DBD Plasma Operating Parameters on the Polymer Surface Modification. Surf. Coat. Technol. 2004, 185, 311–320.
- Walsh, J.L.; Iza, F.; Janson, N.B.; Law, V.J.; Kong, M.G. Three Distinct Modes in a Cold Atmospheric Pressure Plasma Jet. J. Phys. Appl. Phys. 2010, 43, 075201.
- Dolci, L.S.; Quiroga, S.D.; Gherardi, M.; Laurita, R.; Liguori, A.; Sanibondi, P.; Fiorani, A.; Calzà, L.; Colombo, V.; Focarete, M.L. Carboxyl Surface Functionalization of Poly(L-Lactic Acid) Electrospun Nanofibers through Atmospheric Non-Thermal Plasma Affects Fibroblast Morphology. Plasma Process. Polym. 2014, 11, 203–213.
- Yildirim, E.D.; Ayan, H.; Vasilets, V.N.; Fridman, A.; Guceri, S.; Sun, W. Effect of Dielectric Barrier Discharge Plasma on the Attachment and Proliferation of Osteoblasts Cultured over Poly(ε-Caprolactone) Scaffolds. Plasma Process. Polym. 2008, 5, 58–66.
- Benedikt, J. Plasma-Chemical Reactions: Low Pressure Acetylene Plasmas. J. Phys. Appl. Phys. 2010, 43, 043001.
- Piel, A. Plasma Physics: An Introduction to Laboratory, Space, and Fusion Plasmas; Springer: Berline, Germany, 2017; ISBN 978-3-319-63427-2.
- Vitchuli Gangadharan, N. Atmospheric Pressure Plasma-Electrospin Hybrid Process for Protective Applications. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2011.
- Ehlbeck, J.; Schnabel, U.; Polak, M.; Winter, J.; von Woedtke, T.; Brandenburg, R.; von dem Hagen, T.; Weltmann, K.-D. Low Temperature Atmospheric Pressure Plasma Sources for Microbial Decontamination. J. Phys. Appl. Phys. 2010, 44, 013002.
- Lu, X.; Reuter, S.; Laroussi, M.; Liu, D. Nonequilibrium Atmospheric Pressure Plasma Jets: Fundamentals, Diagnostics, and Medical Applications; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-429-62287-8.
- Razzak, M.A.; Takamura, S.; Uesugi, Y. Effects of Radio-Frequency Driving Power, Gas Pressure, and Nitrogen Seeding on the Transition Dynamics in Argon Inductively Coupled Plasmas. J. Appl. Phys. 2004, 96, 4771–4776.
- Mizeraczyk, J.; Jasiński, M.; Zakrzewski, Z. Hazardous Gas Treatment Using Atmospheric Pressure Microwave Discharges. Plasma Phys. Control. Fusion 2005, 47, B589.
- Çınar, K. Design and Construction of a Microwave Plasma Ion Source. Master’s Thesis, Middle East Technical University, Ankara, Turkey, 2011.
- Cogollo, M.; Balsalobre, M.P.; Diaz Lantada, A.; Puago, H. Design and Experimental Evaluation of Innovative Wire-to-Plane Fins’ Configuration for Atmosphere Corona-Discharge Cooling Devices. Appl. Sci. 2020, 10, 10.
- Riba, J.-R.; Morosini, A.; Capelli, F. Comparative Study of AC and Positive and Negative DC Visual Corona for Sphere-Plane Gaps in Atmospheric Air. Energies 2018, 11, 2671.
- Rotan, M.; Zhuk, M.; Glaum, J. Activation of Ferroelectric Implant Ceramics by Corona Discharge Poling. J. Eur. Ceram. Soc. 2020, 40, 5402–5409.
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric Pressure Plasmas: A Review. Spectrochim. Acta B At. Spectrosc. 2006, 61, 2–30.
- Dobslaw, C.; Glocker, B. Plasma Technology and Its Relevance in Waste Air and Waste Gas Treatment. Sustainability 2020, 12, 8981.
- Popelka, A.; Khanam, P.N.; AlMa’adeed, M. Surface Modification of Polyethylene/Graphene Composite Using Corona Discharge. J. Phys. Appl. Phys. 2018, 51.
- Pothanamkandathil, V.; Singh, R.; Philip, L.; Ramanujam, S. Effect of Recycling Overhead Gases on Pollutants Degradation Efficiency in Gas-Phase Pulsed Corona Discharge Treatment. J. Environ. Chem. Eng. 2018, 6, 923–929.
- Ajo, P.; Kornev, I.; Preis, S. Pulsed Corona Discharge in Water Treatment: The Effect of Hydrodynamic Conditions on Oxidation Energy Efficiency. Ind. Eng. Chem. Res. 2015, 54, 7452–7458.
- Pontiga, F.; Hadji, K.; Guemou, M.; Yanallah, K.; Fernández-Rueda, A.; Moreno, H. Ozone Production by Corona Discharge Using Ahollow Needle-Plate Electrode System. In Proceedings of the 20th International Conference on Gas Discharges and their Application, Orleans, France, 6–11 July 2014.
- Bussiahn, R.; Brandenburg, R.; Gerling, T.; Kindel, E.; Lange, H.; Lembke, N.; Weltmann, K.-D.; von Woedtke, T.; Kocher, T. The Hairline Plasma: An Intermittent Negative Dc-Corona Discharge at Atmospheric Pressure for Plasma Medical Applications. Appl. Phys. Lett. 2010, 96, 143701.
- Brandenburg, R. Dielectric Barrier Discharges: Progress on Plasma Sources and on the Understanding of Regimes and Single Filaments. Plasma Sources Sci. Technol. 2017, 26, 053001.
- Kogelschatz, U. Dielectric-Barrier Discharges: Their History, Discharge Physics, and Industrial Applications. Plasma Chem. Plasma Process. 2003, 23, 1–46.
- Subedi, D.P.; Joshi, U.M.; Wong, C.S. Dielectric Barrier Discharge (DBD) Plasmas and Their Applications. In Plasma Science and Technology for Emerging Economies: An AAAPT Experience; Rawat, R.S., Ed.; Springer: Singapore, Singapore, 2017; pp. 693–737. ISBN 978-981-10-4217-1.
- Xu, X. Dielectric Barrier Discharge—Properties and Applications. Thin Solid Films 2001, 390, 237–242.
- Massines, F.; Gherardi, N.; Naudé, N.; Ségur, P. Recent Advances in the Understanding of Homogeneous Dielectric Barrier Discharges. Eur. Phys. J. Appl. Phys. 2009, 47, 22805.
- Ráhel\textquotesingle, J.; Sherman, D.M. The Transition from a Filamentary Dielectric Barrier Discharge to a Diffuse Barrier Discharge in Air at Atmospheric Pressure. J. Phys. Appl. Phys. 2005, 38, 547–554.
- Liu, C.; Park, D.; Friedman, G.; Fridman, A.; Dobrynin, D. Uniform Dielectric Barrier Discharge Generation in Atmospheric Air Using Nano- Second Pulses: Fast Imaging, Spectroscopy, and Production of Active Species. In Proceedings of the 21st International Symposium on Plasma Chemistry (ISPC 21), Queensland, Australia, 4–9 August 2013.
- Borin, D.; Sbaizero, O.; Scuor, N. Application of a Dielectric Barrier Discharge Plasma for Heating Plastic Materials. Plasma Res. Express 2019, 1, 025009.
- Wagner, H.-E.; Brandenburg, R.; Kozlov, K.V.; Sonnenfeld, A.; Michel, P.; Behnke, J.F. The Barrier Discharge: Basic Properties and Applications to Surface Treatment. Vacuum 2003, 71, 417–436.
- Ghomi, H.; Navab Safa, N.; Ghasemi, S. Investigation on a DBD Plasma Reactor. IEEE Trans. Plasma Sci. 2011, 39, 2104–2105.
- Feizollahi, E.; Misra, N.N.; Roopesh, M.S. Factors Influencing the Antimicrobial Efficacy of Dielectric Barrier Discharge (DBD) Atmospheric Cold Plasma (ACP) in Food Processing Applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 666–689.
- Martines, E. Special Issue “Plasma Technology for Biomedical Applications”. Appl. Sci. 2020, 10, 1524.
- Fanelli, F.; Fracassi, F. Atmospheric Pressure Non-Equilibrium Plasma Jet Technology: General Features, Specificities and Applications in Surface Processing of Materials. Surf. Coat. Technol. 2017, 322, 174–201.
- Sohbatzadeh, F.; Samadi, O.; Siadati, S.; Etaati, G.; Asadi, E.; Safari, R. Development of a Radio Frequency Atmospheric Pressure Plasma Jet for Diamond-like Carbon Coatings on Stainless Steel Substrates. Appl. Phys. A 2016, 122.
- Winter, J.; Brandenburg, R.; Weltmann, K.-D. Atmospheric Pressure Plasma Jets: An Overview of Devices and New Directions. Plasma Sources Sci. Technol. 2015, 24, 064001.
- Gomathi, N.; Chanda, A.K.; Neogi, S. Atmospheric Plasma Treatment of Polymers for Biomedical Applications. In Atmospheric Pressure Plasma Treatment of Polymers; John Wiley & Sons: Weinheim, Germany, 2013; pp. 199–215. ISBN 978-1-118-74730-8.
- Penkov, O.V.; Khadem, M.; Lim, W.-S.; Kim, D.-E. A Review of Recent Applications of Atmospheric Pressure Plasma Jets for Materials Processing. J. Coat. Technol. Res. 2015, 12, 225–235.
- Bernhardt, T.; Semmler, M.L.; Schäfer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxid. Med. Cell. Longev. 2019, 2019.
- Alves, P.; Pinto, S.; Ferreira, P.; Kaiser, J.-P.; Bruinink, A.; de Sousa, H.C.; Gil, M.H. Improving Cell Adhesion: Development of a Biosensor for Cell Behaviour Monitoring by Surface Grafting of Sulfonic Groups onto a Thermoplastic Polyurethane. J. Mater. Sci. Mater. Med. 2014, 25, 2017–2026.
- Gozutok, M.; Baitukha, A.; Arefi-Khonsari, F.; Sasmazel, H.T. Novel Thin Films Deposited on Electrospun PCL Scaffolds by Atmospheric Pressure Plasma Jet for L929 Fibroblast Cell Cultivation. J. Phys. Appl. Phys. 2016, 49, 474002.
- Surucu, S.; Sasmazel, H.T. DBD Atmospheric Plasma-Modified, Electrospun, Layer-by-Layer Polymeric Scaffolds for L929 Fibroblast Cell Cultivation. J. Biomater. Sci. Polym. Ed. 2016, 27, 111–132.
- Ozkan, O.; Turkoglu Sasmazel, H. Dielectric Barrier Discharge and Jet Type Plasma Surface Modifications of Hybrid Polymeric Poly (ε-Caprolactone)/Chitosan Scaffolds. J. Biomater. Appl. 2018, 32, 1300–1313.
- Navaneetha Pandiyaraj, K.; Arun Kumar, A.; RamKumar, M.C.; Deshmukh, R.R.; Bendavid, A.; Su, P.-G.; Uday Kumar, S.; Gopinath, P. Effect of Cold Atmospheric Pressure Plasma Gas Composition on the Surface and Cyto-Compatible Properties of Low Density Polyethylene (LDPE) Films. Curr. Appl. Phys. 2016, 16, 784–792.
- Šrámková, P.; Zahoranová, A.; Kelar, J.; Kelar Tučeková, Z.; Stupavská, M.; Krumpolec, R.; Jurmanová, J.; Kováčik, D.; Černák, M. Cold Atmospheric Pressure Plasma: Simple and Efficient Strategy for Preparation of Poly(2-Oxazoline)-Based Coatings Designed for Biomedical Applications. Sci. Rep. 2020, 10, 9478.
- Lee, J.-H.; Kwon, J.-S.; Om, J.; Kim, Y.-H.; Choi, E.-H.; Kim, K.-M.; Kim, K.-N. Cell Immobilization on Polymer by Air Atmospheric Pressure Plasma Jet Treatment. Jpn. J. Appl. Phys. 2014, 53, 086202.
- Ebnesajjad, S. Chapter 9—Plasma Treatment of Polymeric Materials. In Surface Treatment of Materials for Adhesive Bonding, 2nd ed.; Ebnesajjad, S., Ed.; William Andrew Publishing: Oxford, NY, USA, 2014; pp. 227–269. ISBN 978-0-323-26435-8.
- Gomathi, N.; Sureshkumar, A.; Neogi, S. RF Plasma-Treated Polymers for Biomedical Applications. Curr. Sci. 2008, 94, 1478–1486.
