Polyploidy means having more than two basic sets of chromosomes. Polyploid plants may be artificially obtained through chemical, physical and biological (2n gametes) methods.
- amphiploidy
- disomic polyploidy
- allopolyploidy
- autopolyploidy
- chromosome sets
- mutation
- forages
- breeding
- polysomic polyploid
- disomic polyploid
1. Introduction
Polyploidy, which occurs among various life forms [1], allows having more than two basic sets of chromosomes. It is a widely distributed phenomenon in wild and cultivated species in agriculture, forests and rangelands [2]. Whole genome duplications take place at very high rate in forages, e.g., 75% of the speciation underwent genome duplications in group of 1200 species [3].
'The remarkable ecological success of the polyploidy prompts breeders to develop new polyploid germplasm with increased economic value [2]. These polyploids could assist on understanding the evolution of various plant species with economic relevance. Polyploids are also used as bridge species to transfer resistant genes from their parental species [4][5]. In contrast to natural polyploidy arising from natural selection [6], induced polyploid (polysomic polyploids, and true or segmental amphidiploids mainly result from the anti-tubulin effect of colchicine ((S)-N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[a]heptalen-7-yl) acetamide; and also of oryzalin (3,5-dinitro-N
,N
-dipropylsulfanilamide), trifluralin (α,α,α-trifluoro-2,6-dinitro-N,N,-dipropyl-
-toluidine) and amiprophos methyl (O-methyl O-(2-nitro-
-tolyl) N-isopropyl phosphoramidothionate) [7].
The use of artificial polyploidy began in 1937 after the discovery and extraction of the natural compound colchicine, which has the ability to inhibit spindle fiber formation to arrest the chromosome, thus causing a failure of chromatid disjunction [8] and of cytokinesis [7]. Spontaneous chromosome set doubling also occurs in plant somatic cells, and gametes at low frequency, which may subsequently result in a polyploid organism [9] by vegetative or seminal propagation, respectively.
Natural and induced polyploid have been widely investigated in crops, trees, ornamentals, forages and medicinal plants [10]. This literature search and synthesis were pursued to address 21st century challenges related to continuous population growth, as well as the global climate change [11]. Forages are widely exploited for their vegetative biomass that may be used as animal feed or for biofuel [12]. Polyploidy is widely distributed phenomenon among forage species [3][13][14]. It may be induced in diploid species to improve biomass productivity and its nutritional characteristics. We review herein various methods for developing polyploid forage germplasm with chemicals and their commercial importance when compared with ancestral and natural polyploid species.
2. Production of Polyploid Plants
Polyploid plants (polysomic polyploids, true, and segmental amphidiploids) can be produced using various chemicals, such as colchicine, amiprophos-methyl, trifluralin or oryzalin; or physical methods such as temperature shock and protoplast fusion [8]. The most common chemical is colchicine obtained from
L., which has an inhibitory effect on the spindle formation, causing a failure during anaphase disjunction [8] and cytokinesis [7], thus resulting in one cell with the doubled chromosome set. Hence, colchicine treatment results in either pure polyploid individuals or impure (mixoploid or aneuploid cells), depending upon the colchicine concentration and exposure time, plant tissues and its development stage, and method for chromosome set doubling. The colchicine solution may be applied to immature buds, resulting in unreduced reproductive cells and, subsequently, producing polyploidy seed at different rates. It is applied to the pedicel of growing immature bud or the inflorescence stalk may be dipped in a growth media solution with low concentration of colchicine (0.01 to 0.1%), depending upon the forage species (
). Cotton swab dipped in colchicine solution of 0.2% for 10 h during 2 consecutive days successfully induced a polysomic tetraploid (12–40%) in guar accessions [15]. Colchicine can also be applied to the callus; i.e., cell culture of meristematic tissues to produce chimeric plants, or seed may be germinated in a colchicine solution [16]. Generally, colchicine (0.1‒1%) has been applied to seeds for 24–48 h depending upon the forage species to target cells undergoing mitosis (
). Growth arrest is the first symptoms of successful application of colchicine (
). Affected cells usually resume division after a lag period of colchicine application. A comparison of euploid series (2×, 4×, 6× and 8×) in thale cress (
L. Heynh.) showed that induced polyploidy had slower growth, enlarged cell size, and decreased the number of cells per leaf blade. Polyploid cells had lower lignin and cellulose but higher pectin and hemicelluloses in the stem [17]. Frequency of polyploidy induction can increase by rising colchicine concentration (
). A high colchicine concentration has been, however, toxic to cell and plant tissues. A low colchicine concentration (0.1%) treatment with relative long duration (48 h) has been effective in increasing the yield of polyploid cells in shoot tips in vitro [18] (
). For example, sorghum (
(L.) Moench.) seed were subjected to 0.2% concentration of colchicine for 48 h and 72 h, yielding 3.3% and 2.3% polysomic tetraploid plants, respectively [19]. A direct application of colchicine by dipping the apical meristem of seedlings produced chimeric tissues where induced polyploidy was limited to a particular layer [20].

Control vs. various treatments of colchicine (0.25–1%) in growth of interspecific maize × teosinte seedlings after treatment of seeds for 24 h (Niazi, I.A.K., permission granted) [21].
Recovery of induced tetraploids using various concentrations of anti-mitotic treatments.
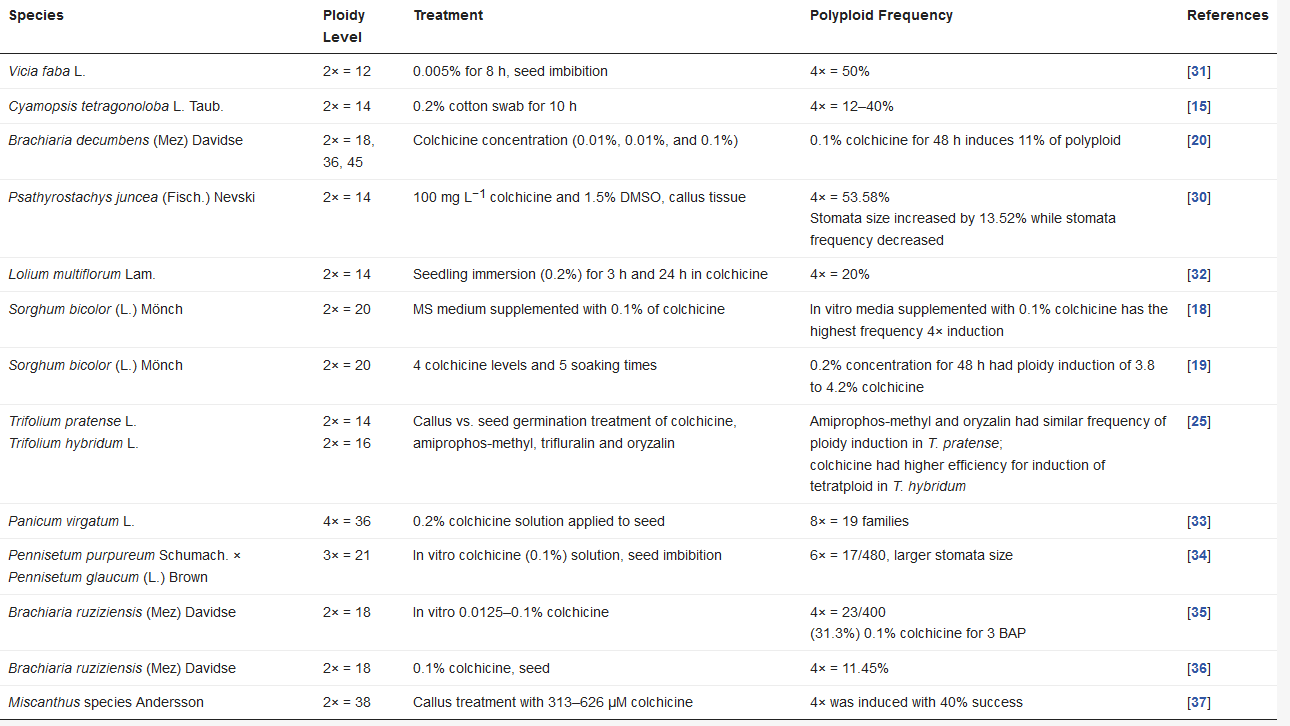
| Species | Ploidy Level | Treatment | Polyploid Frequency | References |
|---|---|---|---|---|
| Vicia faba L. | 2× = 12 | 0.005% for 8 h, seed imbibition | 4× = 50% | [22] |
| Cyamopsis tetragonoloba L. Taub. | 2× = 14 | 0.2% cotton swab for 10 h | 4× = 12–40% | [15] |
| Brachiaria decumbens (Mez) Davidse | 2× = 18, 36, 45 | Colchicine concentration (0.01%, 0.01%, and 0.1%) | 0.1% colchicine for 48 h induces 11% of polyploid | [20] |
| Psathyrostachys juncea (Fisch.) Nevski | 2× = 14 | 100 mg L−1 colchicine and 1.5% DMSO, callus tissue | 4× = 53.58% Stomata size increased by 13.52% while stomata frequency decreased |
[23] |
| Lolium multiflorum Lam. | 2× = 14 | Seedling immersion (0.2%) for 3 h and 24 h in colchicine | 4× = 20% | [24] |
| Sorghum bicolor (L.) Mönch | 2× = 20 | MS medium supplemented with 0.1% of colchicine | In vitro media supplemented with 0.1% colchicine has the highest frequency 4× induction | [18] |
| Sorghum bicolor (L.) Mönch | 2× = 20 | 4 colchicine levels and 5 soaking times | 0.2% concentration for 48 h had ploidy induction of 3.8 to 4.2% colchicine | [19] |
| Trifolium pratense L. Trifolium hybridum L. |
2× = 14 2× = 16 |
Callus vs. seed germination treatment of colchicine, amiprophos-methyl, trifluralin and oryzalin | Amiprophos-methyl and oryzalin had similar frequency of ploidy induction in T. pratense; colchicine had higher efficiency for induction of tetratploid in T. hybridum |
[25] |
| Panicum virgatum L. | 4× = 36 | 0.2% colchicine solution applied to seed | 8× = 19 families | [26] |
| Pennisetum purpureum Schumach. × Pennisetum glaucum (L.) Brown | 3× = 21 | In vitro colchicine (0.1%) solution, seed imbibition | 6× = 17/480, larger stomata size | [27] |
| Brachiaria ruziziensis (Mez) Davidse | 2× = 18 | In vitro 0.0125–0.1% colchicine | 4× = 23/400 (31.3%) 0.1% colchicine for 3 BAP |
[28] |
| Brachiaria ruziziensis (Mez) Davidse | 2× = 18 | 0.1% colchicine, seed | 4× = 11.45% | [29] |
| Miscanthus species Andersson | 2× = 38 | Callus treatment with 313–626 µM colchicine | 4× was induced with 40% success | [30] |
Polyploid plants can ensue from somatic cell fusion [31]. This method has been used to fuse protoplast of the same species (polysomic polyploidy) or from two species (amphiploidy or disomic polyploidy) in an electric shock chamber without reduction division, thus obtaining amphidiploid forage species [32].
In vitro induction of polyploidy was successfully achieved by culturing the nodal segments or callus in tissue culture media supplemented with a low concentration of colchicine. This method induces high polyploidy frequency in various species (
). Although other chromosome doubling agent such as, oryzalin had a higher frequency of induced polyploidy than colchicine [33], it inhibited callus growth and seedling regeneration. Liquid medium was more effective in inducing polyploidy than semi solid media [7][33]. A concentration of 5 µm oryzalin was more effective than colchicine and trifluralin in inducing chromosome doubling of calli obtained from interspecific hybrid plants of elephant grass × pearl millet [34]. In vitro treatment of red clover (
L.) yielded 55.5% more polysomic tetraploids and 1.9 times fewer chimeric individuals than imbibition of seed in a colchicine solution [25][35]. Colchicine, amiprophosmethyl and oryzalin produced similar frequency of polysomic tetraploid (31–47%) and chimeric plants (14–22%) in
[25]. Colchicine (0.015% in tissue culture media) was more effective than oryzalin in inducing polysomic tetraploidy (16%) in peanut clover (
Poir.) [36].
The demand for pure polyploids with different ploidy levels obtained after chromosome doubling needs screening, selection and periodic evaluation of the ploidy level stability of the natural and induced [37]. Nowadays, the main method used for this purpose is flow cytometry [8][38], which increases the efficiency of ploidy level determination and nuclear genome size measurement. With flow cytometry large numbers of individuals are quickly evaluated in only one day using a quantitative, rapid, reliable and reproducible method [11]. This powerful screening tool for ploidy level has been very useful in breeding programs. However, chromosome counting, which is performed through microscopic observation of the metaphase cells, has been applied to confirm the chromosome number [8][37][38], and to detect eventual aneuploids. Besides these direct tools, indirect analyses have also been accomplished. The effects of induced polyploidy on forage species were noted as an increase in the stomatal area and a decrease in the stomatal frequency. These changes in leaf anatomy arose after expanding cell volume due to increasing nuclear DNA content [23].
References
- Chen, Z.J. Molecular mechanisms of polyploidy and hybrid vigor. Trends Plant Sci. 2010, 15, 57–71.
- Shimizu-Inatsugi, R.; Terada, A.; Hirose, K.; Kudoh, H.; Sese, J.; Shimizu, K.K. Plant adaptive radiation mediated by polyploid plasticity in transcriptomes. Mol. Ecol. 2017, 26, 193–207.
- Estep, M.C.; McKain, M.R.; Diaz, D.V.; Zhong, J.; Hodge, J.G.; Hodkinson, T.R.; Layton, D.J.; Malcomber, S.T.; Pasquet, R.; Kellogg, E.A. Allopolyploidy, diversification, and the Miocene grassland expansion. Proc. Natl. Acad. Sci. USA 2014, 111, 15149–15154.
- Randhawa, M.S.; Singh, R.P.; Dreisigacker, S.; Bhavani, S.; Huerta-Espino, J.; Rouse, M.N.; Sandoval-Sanchez, M. Identification and validation of a common stem rust resistance locus in two bi-parental populations. Front. Plant Sci. 2018, 9, 1788.
- Rao, S.; Tian, Y.; Xia, X.; Li, Y.; Chen, J. Chromosome doubling mediates superior drought tolerance in Lycium ruthenicum via abscisic acid signaling. Hort. Res. 2020, 7, 40.
- Te Beest, M.; Le Roux, J.J.; Richardson, D.M.; Brysting, A.K.; Suda, J.; Kubešová, M.; Pyšek, P. The more the better? The role of polyploidy in facilitating plant invasions. Annal. Bot. 2012, 109, 19–45.
- Venial, L.R.; Mendonça, M.A.C.; Amaral-Silva, P.M.; Canal, G.B.; Passos, A.B.R.J.; Ferreira, A.; Soares, T.C.B.; Clarindo, W.R. Autotetraploid Coffeacanephora and auto-alloctaploid Coffeaa rabicafrom in vitro chromosome set doubling: New germplasms for Coffea. Front. Plant Sci. 2020, 11, 154.
- Dhooghe, E.; Van Laere, K.; Eeckhaut, T.; Leus, L.; Van Huylenbroeck, J. Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult. 2011, 104, 359–373.
- Bretagnolle, F.A.; Thompson, J.D. Gametes with the somatic chromosome number: Mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytol. 1995, 129, 1–22.
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2016, 243, 281–296.
- Ciprian-Salcedo, G.C.; Jimenez-Davalos, J.; Zolla, G. Flow-cytometry applications in plant breeding. Rev. Perua. Biol. 2020, 27, 79–84.
- Rauf, S.; Sienkiewicz-Paderewska, D.; Malinowski, D.P.; Hussain, M.M.; Niazi, I.A.K.; Kausar, M. Forages: Ecology, breeding objectives and procedure. In Advances in Plant Breeding Strategies: Agronomic, Abiotic and Biotic Stress Traits; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: Cham, Switzerland, 2016; pp. 149–201.
- Stebbins, G.L. The origin of the complex of Bromus carinatus and its phylogeographic implications. Gray Herb. Harv. Univ. 1947, 165, 42–55.
- Stebbins, G.L. Chromosomes and evolution in the genus Bromus (Gramineae). Bot. Jahrb. Syst. Pflanzengesch. Pflanzengeogr. 1981, 102, 359–379.
- Rao, S.R.; Kumar, A.; Purohit, J.; Khedasana, R.; Bewal, S. Cytogenetical investigations in colchicine induced tetraploids of Cyamopsis tetragonoloba L. Czech J. Genet. Plant Breed. 2010, 45, 143–154.
- Niazi, I.A.K.; Rauf, S.; Teixeira da Silva, J.A.; Iqbal, Z.; Munir, H. Induced polyploidy in inter-subspecific maize hybrids to reduce heterosis breakdown and restore reproductive fertility. Grass Forage Sci. 2015, 70, 682–694.
- Corneillie, S.; De Storme, N.; Van Acker, R.; Fangel, J.U.; De Bruyne, M.; De Rycke, R.; Boerjan, W. Polyploidy affects plant growth and alters cell wall composition. Plant Physiol. 2019, 179, 74–87.
- Ardabili, G.S.; Zakaria, R.A.; Zare, N. In vitro induction of polyploidy in Sorghum bicolor L. Cytologia 2015, 80, 495–503.
- Poosamart, W.; Siniri, N.; Simia, S. Effects of colchicine concentration level and soaking period to induce polyploid mutation on five forage sorghum (Sorghum bicolor L. Moench) cultivars. J. Mahanakorn Veter. Med. 2015, 10, 99–110.
- Simioni, C.; Valle, C.B. Meiotic analysis in induced tetraploids of Brachiari Adecumbens Stapf. Crop Breed. Appl. Biotechnol. 2011, 11, 43–49.
- Niazi, I.A.K. Evaluation of Zea mays × Zea mexicana for High Fodder Yield. Ph.D. Thesis, University of Sargodha, Sargodha, Pakistan, 2016.
- Joshi, P.; Verma, R.C. High frequency production of colchicine induced autotetraploids in faba bean (Viciafaba L.). Cytologia 2004, 69, 141–147.
- Yun, L.; Yun, J.; Li, J.; Zheng, L.; Zhao, W.; Qi, L. Callus polyploidy induction and identification of Russian wild ryegrass. Acta Pratac. Sin. 2010, 19, 126–131.
- Pereira, R.C.; Ferreira, M.T.M.; Davide, L.C.; Pasqual, M.; Mittelmann, A.; Techio, V.H. Chromosome duplication in Lolium multiflorum Lam. Crop Breed. Appl. Biotechnol. 2014, 14, 251–255.
- Dabkevičienė, G.; Statkevičiūtė, G.; Mikaliūnienė, J.; Norkevičienė, E.; Kemešytė, V. Production of Trifolium pratense L. and T. hybridum L. tetraploid populations and assessment of their agrobiological charac-teristics. Zemdir. Agric. 2016, 103, 377–384.
- Yoon, S.; Aucar, S.; Hernlem, B.J.; Edme, S.; Palmer, N.; Sarath, G.; Mitchell, R.; Blumwald, E.; Tobias, C.M. Generation of octaploid switchgrass by seedling treatment with mitotic inhibitors. BioEnergy Res. 2017, 10, 344–352.
- Campos, J.M.S.; Davide, L.C.; Salgado, C.C.; Santos, F.C.; Costa, P.N.; Silva, P.S.; Pereira, A.V. In vitro induction of hexaploid plants from triploid hybrids of Pennisetum purpureum and Pennisetum glaucum. Plant Breed. 2009, 128, 101–104.
- Ishigaki, G.; Gondo, T.; Suenaga, K.; Akashi, R. Induction of tetraploid ruzigrass (Brachiaria ruziziensis) plants by colchicine treatment of in vitro multiple-shoot clumps and seedlings. Grassl. Sci. 2009, 55, 164–170.
- Timbó, A.L.D.O.; Souza, P.N.D.C.; Pereira, R.C.; Nunes, J.D.; Pinto, J.E.B.P.; SouzaSobrinho, F.D.; Davide, L.C. Obtaining tetraploid plants of ruzigrass (Brachiaria ruziziensis). R. Bras. Zootec. 2014, 43, 127–131.
- Głowacka, K.; Jeżowski, S.; Kaczmarek, Z. In vitro induction of polyploidy by colchicine treatment of shoots and preliminary characterisation of induced polyploids in two Miscanthus species. Ind. Crop. Prod. 2010, 32, 88–96.
- De Souza-Kaneshima, A.M.; Simioni, C.; Felismino, M.F.; Mendes-Bonato, A.B.; Risso-Pascotto, C.; Pessim, C.; Pagliarini, M.S.; Do Valle, C.B. Meiotic behaviour in the first interspecific hybrids between Brachiaria brizantha and Brachiaria decumbens. Plant Breed. 2010, 129, 186–191.
- Pagliarini, M.S.; Carneiro Vieira, M.L.; Borges do Valle, C. Meiotic Behavior in Intra-and Interspecific Sexual and Somatic Polyploid Hybrids of Some Tropical Species. In Meiosis Molecular Mechanisms and Cytogenetic Diversity. 2012. Available online: (accessed on 28 October 2019).
- Yu, C.Y.; Kim, H.S.; Rayburn, A.L.; Widholm, J.M.; Juvik, J.A. Chromosome doubling of the bioenergy crop, Miscanthus× giganteus. GCB Bioenergy 2009, 1, 404–412.
- Faleiro, F.G.; Kannan, B.; Altpeter, F. Regeneration of fertile, hexaploid, interspecific hybrids of elephant grass and pearl millet following treatment of embryogenic calli with antimitotic agents. Plant Cell Tissue Organ Cult. 2016, 124, 57–67.
- Dabkevičienė, G.; Kemešytė, V.; Statkevičiūtė, G.; Lemežienė, N.; Brazauskas, G. Autopolyploids in fodder grass breeding: Induction and field performance. Span. J. Agric. Res. 2017, 15, 20.
- Castillo, A.; Lopez Carro, B.; Dalla Rizza, M.; Reyno, R. Use of in vitro methods to induce autotetraploids in the native forage legume Trifolium polymorphum. In VII International Symposium on Production and Establishment of Micropropagated Plants; ISHS: Lavras, Minas Gerais, Brazil, 2017; pp. 73–80.
- Silva, A.J.; Carvalho, C.R.; Clarindo, W.R. Chromosome set doubling and ploidy stability in synthetic auto- and allotetraploid of Eucalyptus: From in vitro condition to the field. Plant Cell Tissue Organ Cult. 2019, 138, 387–394.
- Dirihan, S.; Terho, P.; Helander, M.; Saikkonen, K. Efficient analysis of ploidy levels in plant evolutionary ecology. Caryologia 2013, 66, 251–256.
