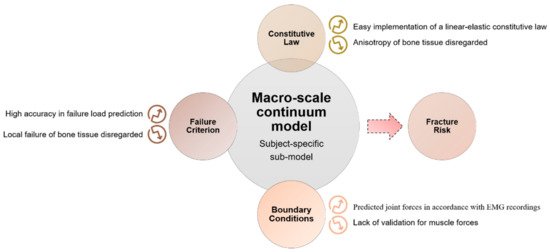
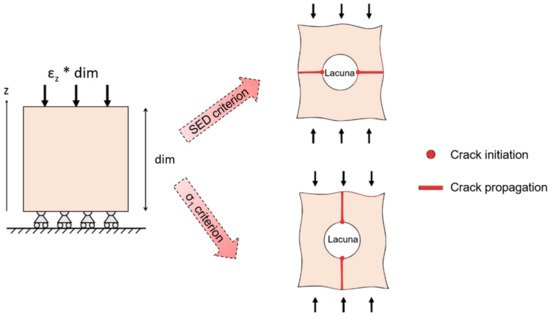
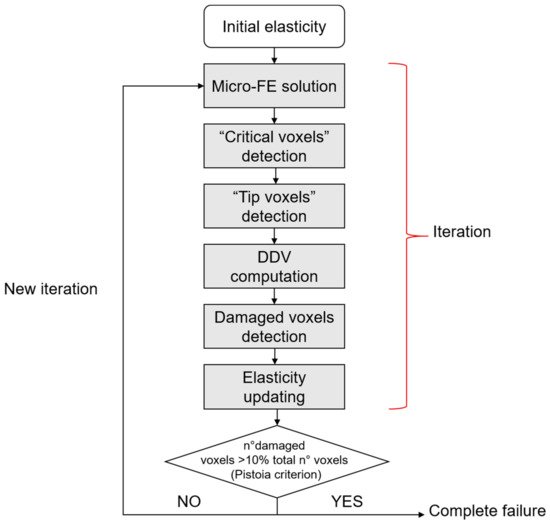
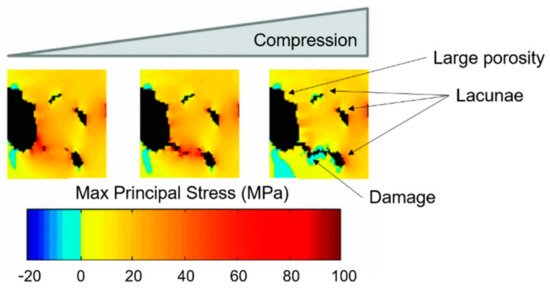
The investigation of bone damage processes is a crucial point to understand the mechanisms of age-related bone fractures. In order to reduce their impact, early diagnosis is key. The intricate architecture of bone and the complexity of multiscale damage processes make fracture prediction an ambitious goal.
- age-related bone fractures
- multiscale imaging
- bone damage
- computational models
- experimental validation
1. Imaging Techniques for Multiscale Damage Assessment and Prediction
Figure 1) to visualize bone morphologies, to assess bone fractures and to predict the fracture risk from the macro-scale to the nano-scale. The macro-architecture is currently evaluated by means of common clinical images. At lower hierarchical levels, the identification of damage processes is more complex and requires higher-resolution techniques.
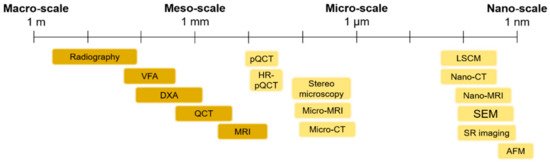
Figure 14.
1.1. Macro- and Meso-Scale Imaging
| Macro- and Meso-Scale Imaging Technique | Brief Description of the Technique | Invasiveness | Outcomes | Spatial Resolution | 2D or 3D | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| In Vitro/In Vivo Application | |||||||
| Radiography | Based on the interaction between a beam of photons (X-rays) directed from a source to a receptor. The atoms of the body prevent, in a percentage dependent on their atomic number, some photons from reaching the receptor, reproducing a “negative” image of the body | No Radiation dose: 40–50 times lower, if compared to computed tomography (CT) scans (e.g., radiographs of the abdomen → 0.25 mGy) [1][39] |
|||||
| Claustrophobia side effect | |||||||
| In vivo | |||||||
1.2. Micro- and Nano-Scale Imaging
| Micro- and Nano-Scale Imaging Technique | Brief Description of the Technique | Invasiveness | Outcomes | Spatial Resolution | 2D or 3D | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| In Vitro/In Vivo Application |
| Estimation of density variation (fracture risk prediction) by means of two indexes: Singh index | ||||
| [ | 2 | ] | [40] for proximal femur and cortical–medullary index [3][41] for hand radiographs | 0.17 mm/pixel → The size of the monitor screens used in digital radiography is sufficient for 35 × 43 cm2 radiographs to be displayed at a resolution of 2048 × 2560 pixels [4 |
| Stereomicroscopy Based on Histological Sections | Histology from the bone tissue is obtained and then the sample is properly treated (fixation, dehydration and clearing, embedding, sectioning, staining and mounting). The histological section is then observed by means of an optical microscope | ] | [ | 42] | 2D | Yes | Clear identification of distal radius fractures | Traditional technique for the visualization of bone microarchitecture | ~1.6 µm [21][58] | [5][43] | 2D | Difficult detection of hip and spine fractures | Bone remodeling assessment [22][59] | Insensitive to changes in Bone Mineral Density (BMD)until 20 to 40% of bone mass lost [5][43] |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Destructive and invasive technique | Limitations related to the bidimensional output images: the three-dimensional features are lost. | High-resolution images (at least 1.4 µm or better) are required to identify and measure individual resorption cavities in the process of bone remodeling | [ | 22][59] | In vivo | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| In vitro | Dual-energy X-ray Absorptiometry (DXA) | Involves the emission of two X-ray beams with different energy levels, that collide with the body of the patient. Once the absorption of the soft tissue has been subtracted, it is possible to determine the absorption of the beam by the bone and therefore the BMD | No | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Micro-Computed Tomography (Micro-CT) and Nano-Computed Tomography (Nano-CT) | Low radiation dose (0.001–0.003 mGy for L-spine, to 0.004 mGy for total body) [6][ |
Micro- and nano-CT scans use radiographs to generate cross-sections of bone, that are generally processed (image reconstruction) to generate a virtual 3D model without destroying the original bone sample | 37] | No Generally, the samples are obtained from surgical wastes that derive from prosthetic treatment | Determination of areal BMD in g/cm2 Calculation of bone mineral content (BMC = BMD × area) Calculation of T-score and Z-score (negative for values under the average BMD), that are numerical indexes for the evaluation of osteoporosis. |
Microarchitectural 3D data for both the cortical and the trabecular sections (tissue volume, bone volume, bone surface, bone volume fraction, bone surface to tissue volume, trabecular/cortical thickness, degree of anisotropy, cortical porosity, etc.) [6][37 | 1 pixel → ≃ 0.56 × 0.56 mm2. (for a Hologic system) [7][44] |
2D | Ease of use of the equipment Standardization Short examination time [8][45] |
No bone architecture detection (no difference between cortical and trabecular bone) Sampling errors Incorrect evaluation in obese patients [6][37] |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| In vivo | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ] | . | Local and global parameters related to the lacunar network are obtained [23][36] | 1.2 µm (micro-CT) ~50–150 nm (nano-CT) |
3D | Large number of obtainable outputs (morphological parameters at different scales) Detailed finite element 3D models could be implemented by using micro-CT images |
Static evaluation of micro-scale features Not suitable for in vivo human evaluation due to the high radiation dose No detection of the canalicular network (insufficient resolution for the micro-CT scans) Nano-CT |
Vertebral Fracture Assessment (VFA) | Special DXA analysis that permits the detection of spinal fractures from a lateral image of the spine | No Lower radiation exposure with respect to spine radiography [9][46] |
Spinal fracture detection [10][47] | Low spatial resolution | 2D | Possibility to add a VFA scan after areal BMD assessment High sensitivity High specificity [11][48] |
Low spatial resolution | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| In vitro | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Peripheral QCT (pQCT) and High-Resolution pQCT (HR-pQCT) | Dedicated CT scanners for the forearm (radius and ulna) and leg (tibia and fibula) | No Low radiation dose (≈0.003 mGy) [6][37] |
Analysis of the trabecular and cortical sections (BMD, bone mineral content and bone geometrical parameter calculation). Acquisition of biomechanical parameters, such as the cross-sectional moment of inertia. Evaluation of the functional muscle–bone unit [24][60]. |
Isotropic voxel size of 82 μm with HR-pQCT | 3D | High precision and accuracy |
In vivo | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Low radiation dose | Applicable for the study of a large number of diseases, especially pediatric (useful in applications where trabecular and cortical sections are affected in a different way) | Evaluation restricted to the appendicular bone | Only transversal data are available for fracture risk prediction Low spatial resolution |
Quantitative Computed Tomography (QCT) | X-ray-based technique that measures BMD. It produces cross-sectional images of X-ray absorption coefficient (measured in Hounsfield units) calibrated to water. It is used to evaluate fracture risk primarily at the lumbar spine and at the hip [12][49] | Medium–high invasiveness Medium–high radiation dose (0.2–0.4 mGy for a spine exam) [13][50] |
True measurement of BMD assessment (areal BMD does not predict if an individual patient will eventually fracture) |
100× higher resolution with respect to conventional radiologic imaging [14][51] | 3D (multiple slices are obtained and then reconstructed) | Fracture risk prediction in patients with scoliosis, obesity, etc. without having artificially high BMD values, as in DXA [15] | High cost [16][53] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| In vivo | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic Resonance Imaging (MRI) | MRIs employ a magnetic field that forces protons in the body to align with that field. When a radiofrequency current is pulsed through the patient, the protons are strained against the pull of the magnetic field. When the radiofrequency field is turned off, the MRI sensors detect the released energy as the protons realign with the magnetic field. The time it takes for the protons to realign, as well as the amount of energy released, changes depending on the environment and the chemical nature of the molecules | No MRI does not use ionizing radiation |
Bone fracture detection Parameters: T2* [17][54] (effective transverse relaxation time) → a function of the density and orientation of the trabeculae [18][55] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| In vivo | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synchrotron Radiation Imaging (SR) | A high-intensity white beam travels around a fixed closed loop. It permits a high level of detail in bone visualization (ultra-structural porosity detection) | No Generally, the samples are obtained from surgical wastes that derive from prosthetic treatment |
Morphological analysis of ultra-structural porosities | Voxel size of 0.9 μm for the white beam [25][61] | 3D | [52]
|