Angiogenesis plays an important role in several physiological and pathological processes. Pharmacological angiogenesis modulation has been robustly demonstrated to achieve clinical benefits in several cancers. Adrenocortical carcinomas (ACC) are rare tumors that often have a poor prognosis. In addition, therapeutic options for ACC are limited. Understanding the mechanisms that regulate adrenocortical angiogenesis along the embryonic development and in ACC could provide important clues on how these processes could be pharmacologically modulated for ACC treatment. In this report, we performed an integrative review on adrenal cortex angiogenesis regulation in physiological conditions and ACC. During embryonic development, adrenal angiogenesis is regulated by both VEGF and Ang-Tie signaling pathways. In ACC, early research efforts were focused on VEGF signaling and this pathway was identified as a good prognostic factor and thus a promising therapeutic target.
- angiogenesis
- adrenal fetal cortex
- adrenocortical carcinoma
1. Introduction
2. Angiogenesis Regulation
Angiogenesis plays a central role in several physiological (e.g., fetal development and wound healing) and pathological processes (e.g., vascular overgrowth for tumor expansion and metastasis) [2][3][4]. Angiogenesis, either in normal or tumor tissues, usually occurs via one or more of the following mechanisms:
- (1)
-
Sprouting angiogenesis, one the most well characterized mechanism leading to angiogenesis, relies on endothelial cells function specification into either tip or stalk cells. Tip cells are derived from the parent vessel, degrade the basement membrane, extend large filopodia which can sense angiogenic factor gradients, such as vascular endothelial growth factor (VEGF), and migrate along the chemotactic paths. In contrast, stalk cells proliferate behind tip cells to form the sprout body, start the process of lumen formation, and connect with neighboring vessels [5][6][7][5,6,7].
- (2)
-
Intussusceptive angiogenesis is a process that consists in the splitting of pre-existing vessels into two new vessels. It starts with the formation of transluminal tissue pillars through the invagination of opposing capillary endothelial cells into the vascular lumen, creating a zone of contact. Commonly, intussusceptive and sprouting angiogenesis are complementary mechanisms [5][8][5,8].
- (3)
-
Recruitment of endothelial progenitor cells and vasculogenesis, a process through which endothelial progenitor cells are recruited in response to several growth factors, cytokines and/or hypoxia-inducible factors. Endothelial progenitor cells differentiate into mature endothelial cells and are incorporated into the angiogenic sprout, thus contributing to new blood vessel formation [4][9][4,9].
- (4)
2.1. VEGF Pathway in Angiogenesis Regulation
In mammals, the VEGF system mainly includes five secreted ligands (VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor) and three primary tyrosine kinase receptors (VEGF-R1, VEGF-R2, VEGF-R3) [13]. The VEGF system also includes the cell-surface proteins, heparan sulfate proteoglycans and neuropilin-1 and -2, which operate as VEGF coreceptors [14][15].
2.2. Ang-Tie Pathway in Angiogenesis Regulation
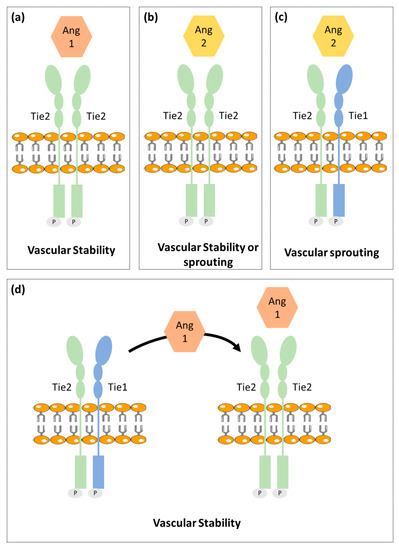
Figure 1.
a
b
c
d
The angiopoietin family includes two type 1 transmembrane protein receptors: Tie1 and Tie2 and four ligands: Ang1, Ang2, Ang3 and Ang4. Ang1 and Ang2 have been identified as the main ligands for Tie receptors, while the Ang3 and Ang4 biological function is still poorly characterized [20][21][22].
Contrary to Tie2, the Tie1 has been less well characterized. Tie1 is considered an orphan receptor and is mainly expressed at vascular bifurcations and branching points, with no yet identified in vivo ligand [28]. It is well known, however, that Tie1 has an important role in vascular development, since its inactivation causes late embryonic lethality and vasculature maturation failure [29][30]. Recent studies proposed that Tie1 forms a complex with Tie2 on the endothelial cell surface and acts as a Tie2 inhibitor [27]. Cells expressing both receptors are responsive to chemotactic signals and able to promote vessel branching and sprouting that is required for angiogenesis. On the other hand, Tie1 is absent in stable and quiescent mature vessels [27].
A mechanistic study indicated Tie1 as being responsible for angiopoientin’s differential function. In mature vessels, as Tie1 is absent, Tie2 can be activated by either Ang1 or Ang2, to promote vessel stability. On active angiogenesis sites, Tie1 and Tie2 form a complex and Ang2 fails to activate Tie2, allowing vessel branching to be promoted. On the other hand, Ang1 is able to dissociate Tie2 from the Tie1-Tie2 complex, activating Tie2 and thus enhancing vascular stability [27][31].
3. Angiogenesis in Normal Adrenal Cortex
3.1. Fetal Adrenal Cortex
Human fetal adrenal (HFA) plays a critical role in fetal maturation and perinatal survival. HFA steroid hormones regulate intrauterine homeostasis and appropriate fetal organ systems maturation [32][33].
Contrary to the adult adrenal cortex that includes three distinct zones: glomerulosa, fasciculata and reticularis; the HFA is primarily composed of two single distinct zones: outer zone or definitive zone and inner zone or fetal zone [33][34]. The definitive zone comprises a narrow band of small cells that exhibit typical characteristics of cells in proliferative state. Definitive zone does not produce steroids until the third trimester. However, as gestation advances, definitive zone cells start to accumulate lipids and resemble steroidogenic active cells. The fetal zone is the largest adrenal cortex zone and consists of large cells that exhibit features characteristic of steroid-secreting cells [33][34][35][36][37]. In ultrastructural studies, a third zone in between definitive zone and fetal zone, named transitional zone, has been described. The transitional zone is composed by cells with intermediate characteristics, but capable to synthetize cortisol and so cells can be considered analogous to fasciculata layer cells of mature adrenal cortex [33][38][39][40][41].
Previous studies have reported that VEGF-A, FGF-2, Ang1, Ang2, and Tie2 are expressed in HFA since midgestation and to have a putative role in adrenal gland angiogenesis [34][42][43].
Ang2, FGF-2 and VEGF-A expression are mainly expressed in the gland periphery suggesting that the HFA periphery is the primary site of angiogenesis, in parallel to cell proliferation [42][43]. Further supporting this hypothesis, a dense network of irregular capillaries was also observed at the HFA periphery [44].
On the contrary, Ang1 is mainly expressed in the fetal zone, suggesting that the inner adrenal zone presents a greater vessel maturity. Tie2, was exclusively identified to be present in endothelial cells throughout the gland [42][43].
Adrenocorticotropic hormone (ACTH), the main regulator of HFA growth and function, also seems to be implicated in angiogenesis control. In vitro studies found that ACTH upregulates VEGF-A, FGF-2 and Ang2 in the HFA, therefore controlling angiogenesis while simultaneously exerting growth and secretion stimulatory actions [42][43][45][46].
Overall, these findings support that the adrenal gland growth, steroidogenesis and blood vessel formation, are synchronized phenomena [42][43][45][46].
3.2. Adult Adrenal Cortex
The adrenal gland is one of the most vascularized organs in adult mammalian organisms. Its developed intrinsic vasculature is required for an efficient secretion of steroid hormones into the systemic blood flow. The adrenal gland is supplied by three different arterial branches derived the abdominal aorta: inferior phrenic artery, middle adrenal artery and renal artery. The arterial blood enters in the adrenal gland and flows centripetally through the adrenal cortex into the adrenal medulla [49][50].
Previous studies have found that adrenocortical cells highly express VEGF-A and EG-VEGF—a VEGF specific of steroidogenic organs, both having been pointed out as important molecules for maintenance of the dense and fenestrated vasculature of the adrenal cortex. This expression also seems to be regulated by ACTH [51][52][53][54][55].
4. Angiogenesis in Adrenocortical Tumors
Adrenocortical tumors (ACT) are common adrenal tumors affecting 3% to 10% of the human population [56]. The majority of ACT are benign non-functioning adrenocortical adenomas (ACA), while malignant ACC are rare with an incidence of 0.7 to 2 per million per year [56]. ACC most often have a poor prognosis and are frequently already metastasized when first diagnosed. ACC pathogenesis is still largely unclear, which results in a lack of biomarkers available for diagnosis and in limited treatment options [57][58].
Table 1.
| Patient Group Comparisons | Results | |||
|---|---|---|---|---|
| VEGF | Patients with ACT vs. Healthy individuals | ↑ VEGF serum levels in patients with ACT [59][60] | ↑ VEGF serum levels in patients with ACT [59,60] |
|
| Aldosterone secreting ACA vs. Non-functioning ACA | ↑ VEGF tumor expression in aldosterone producing ACA [61] | |||
| Cortisol secreting ACA vs. Aldosterone secreting ACA | ↑ VEGF serum levels patients with cortisol secreting ACA [60] | |||
| ACC vs. Normal adrenal glands | ↑ VEGF expression in ACC [61][62] | ↑ VEGF expression in ACC [61,62] |
||
| ACC vs. ACA | ↑ VEGF serum levels in ACC ↑ VEGF tumor expression in ACC [59] | [59 | [61][63][64] | ↑ VEGF serum levels in ACC ↑ VEGF tumor expression in ACC ,61,63,64] |
| Patients with recurrent ACC vs. Patients with non-recurrent ACC | ↑ VEGF serum levels in recurrent ACC ↑ VEGF tumor expression in recurrent ACC [60][63] | ↑ VEGF serum levels in recurrent ACC ↑ VEGF tumor expression in recurrent ACC [60,63] |
||
| Localized ACC vs. Invasive ACC | No difference in VEGF tumor expression [63] |
|||
| VEGF-R2 | ACC vs. Normal adrenal glands | ↑ VEGF-R2 tumor expression in ACC [62] |
||
| ACC vs. ACA | ↑ VEGF-R2 tumor expression in ACC [64] |
Patients with ACT were found to present higher VEGF serum levels as compared to healthy controls [59][60]. In addition, Kolomecki et al. demonstrated that VEGF serum levels were significantly higher in patients with non-functioning malignant tumors than in patients with non-functioning ACA. Noteworthy, VEGF serum levels in patients with ACC were shown to decrease after tumor surgical resection and increase in patients who experienced tumor recurrence [59]. de Fraipont et al. found that cytosolic VEGF-A concentrations were higher in ACC when compared to ACA, although not being significantly different when localized and more invasive ACC were compared [63]. Nevertheless, cytosolic VEGF-A concentrations were higher in recurrent as compared to non-recurrent ACC after primary tumor resection [63].
Tumor VEGF expression was also found to be higher in ACC as compared to normal adrenal glands and ACA [61][62][64]. VEGF receptor 2 tumor expression was also found to be higher in ACC when compared with ACA and normal adrenal glands [62][64].
Other studies reported that although no differences in vascular density were noticed when ACC, ACA and normal adrenal glands were compared, blood vessels perimeter and area were higher in ACC when compared to ACA [65][66]. In addition, endothelial cell proliferation was higher in ACC [66].
New prognostic and diagnostic markers are needed to improve ACC clinical practice. As described in this section, the usefulness of angiogenic factors for ACC diagnosis and/or prognosis was already investigated. From those, VEGF was the one with more consistent and replicable results, being increased in ACC when compared with ACA [59][61][63][64], in particular in the recurrent malignant tumors [60][63]. However, due to the rarity of ACC, the number of patients included in each study is small. So, in the future, to validate this result, multi-center studies are needed to increase the samples/participants’ number and to uniformize the methodological approach to analyze the VEGF tumors expression in ACT. Stratified analysis according to tumors functionality are needed since in previous studies, it showed to influence VEGF levels.
