Adrenocortical carcinoma (ACC) is a rare endocrine malignancy with limited treatment options in the advanced stages.
- immunotherapy
- pembrolizumab
- adrenocortical carcinoma
- anti-PD-L1
1. Introduction
Adrenocortical carcinoma (ACC) is a rare endocrine malignancy with an annual incidence of 0.5–2 cases per million people [1,2][1][2]. More than half of ACC patients present locally advanced or metastatic disease [3]. The prognosis in advanced stages is poor, with a 5-year survival of 15% [4]. Moreover, in this situation, there are limited treatment options and evidence is quite scarce since although some prospective clinical studies have been carried out [5[5][6][7],6,7], most recommendations for ACC treatment are derived from retrospective studies. Mitotane is the only approved and consensually recommendable drug for treatment of advanced ACC [4]. Metastasectomy may be benefiting with a proper patient selection and when surgery is performed by high-volume surgeons practicing at high-volume centers [8]. Currently, systemic chemotherapy—mostly based on combination with etoposide and doxorubicin plus mitotane (EDP-M scheme)—is the most validated treatment option in advanced ACC with unfavorable prognostic parameters [4]. However, it has suboptimal efficacy and short-lived duration of disease control [9]. Radiotherapy is mostly palliative to treat selected sites of symptomatic or high-risk metastases [10]. Thus, the treatment of patients with advanced ACC refractory to “standard” therapies remains challenging. Obviously, in this setting patients should be discussed in a multidisciplinary expert team meeting with experience in care for patients with this rare disease. Apart from this, the enrolment in clinical trials based on an individual basis should be considered [4]. In this way, the collection of biological material is important with the aim of defining potential biomarkers of treatment response in the era of personalized medicine. The specific molecular alteration profiles of ACC may represent targetable events by the use of already developed or newly designed drugs enabling a better and more efficacious management of the ACC patient [11]. Molecular studies have nominated several genes as potential drivers involved in sporadic ACC tumorigenesis, including insulin-like growth factor 2 (IGF2) [12,13][12][13], β-catenin (CTNNB1) [14], and TP53 [15], among others. However, their role as a predictors of treatments response has been poorly investigated [16,17,18,19][16][17][18][19].
Regarding clinical trials investigating experimental therapies, with second-line cytotoxic regimens [9,20,21,22][9][20][21][22] the response rates are lower than 10% and median progression-free survival (PFS) is below 4 months. Neither mTOR targeting drugs nor tyrosine kinase inhibitors (TKI) are effective to avoid the early disease progression [23,24][23][24]. Furthermore, although targeting IGF2/IGF receptor signaling seemed a promising approach based on pathophysiology, the large phase III GALACCTIC trial with linsitinib has not demonstrated any improvement in progression-free or overall survival [25]. Nevertheless, a recent preclinical study suggests that the addition of mTOR inhibitors to linsitinib may increase the antiproliferative effects of linsitinib used in monotherapy [26], although it has not been clinically demonstrated. On the other hand, immunotherapy is the latest revolution in cancer therapy. However, data about the efficacy of this therapy in ACC are limited as only five clinical trials with four immune checkpoint inhibitors for the treatment of advanced ACC have been carried out. Moreover, the results regarding its efficacy are heterogeneous [27,28,29,30,31][27][28][29][30][31]. Nevertheless, the identification of molecular or immunological predictive factors of response may improve the antitumor immune response with these therapies [32,33,34,35][32][33][34][35]. On the other hand, mechanisms of immune resistance could be responsible for the initial disappointing results, so different strategies to overcome resistance should be considered [36,37][36][37].
2. Molecular Background of Adrenocortical Carcinoma
The molecular mechanisms underlying ACC onset and progression remain to be fully elucidated. Two major studies of the molecular basis of ACC—Assie et al. [12] and Zheng et al. [13]—demonstrated that loss of heterozygosity of the IGF2 locus is a common event in ACC leading to upregulation of IGF2/IGF1R signaling. Moreover, ACC shows recurrent somatic alterations facilitating rapid cell cycling, telomere maintenance, and constitutive Wnt/β-catenin and protein kinase A (PKA) signaling, in addition to those involved in chromatin remodeling, transcription, and translation [12[12][13],13], and exhibits frequent copy number alterations [13,38][13][38] (Figure 1 and Table 1).
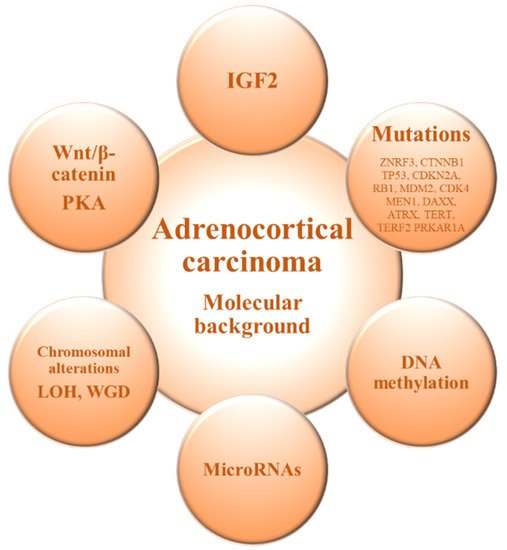
Figure 1. Molecular alterations in adrenocortical carcinoma. IGF2: Insulin-like growth factor 2; LOH: Loss of heterozygosity; PKA: Protein kinase A; WGD: Whole-genome doubling.
The genomics of the adrenocortical tumors can be useful for differential diagnosis to discriminate between benign and malignant forms [11,39][11][39]. It has been shown that ACC and adrenocortical adenoma (ACA) show a differential gene expression profile, and genes involved in processes such as cell cycle or immunity are deregulated in ACC compared with ACA [15,40,41][15][40][41]. Among them, IGF2 is the most up-regulated gene in the malignant forms and previous studies have confirmed an overexpression of IGF2 in 90% of ACCs [12,13,15,41][12][13][15][41]. However, Heaton et al. [42] demonstrated that IGF2 overexpression probably requires additional pathways (e.g., Wnt/β-catenin signaling) for adrenocortical tumorigenesis. Gene expression profiling by transcriptome analysis identified somatic inactivating mutations of the tumor suppressor gene TP53 and activating mutations of the proto-oncogene β-catenin (CTNNB1) as frequent mutations in ACC, which seemed to be mutually exclusive and were observed only in the poor-outcome ACC group [43]. On the other hand, unsupervised clustering analysis identified two groups of malignant tumors with very different outcome based on the combined expression of PINK1 with DLG7 or BUB1B, which was the best predictor of disease-free and overall survival, respectively [44].
In terms of DNA methylation, previous studies have demonstrated that hypo and hyper-methylation alter gene expression [45,46][45][46]. Genomic studies have shown that ACCs are globally hypomethylated compared with ACAs, mainly in intergenic regions [47,48][47][48]. On the other hand, it has also been observed hypermethylated CpG islands in the promoter regions in ACC, with a possible downregulation of tumor suppressor genes [48,49][48][49]. The methylation levels of CpG islands correlates with some prognostic features and, in particular, a hypermethylated profile is associated with a poorer prognosis of ACC [12,13,49,50][12][13][49][50]. In this scenario, an altered DNA methylation status of the IGF2 locus has been associated with ACC tumorigenesis [51].
The microRNA (miRNAs) expression profile has also been shown to discriminate ACC from ACA. A deregulated expression of miRNAs has been demonstrated to alter gene expression, thus providing new tools for cancer diagnosis and prognosis [52,53,54][52][53][54]. Several miRNAs are differentially expressed in ACC compared to ACA, highlighting the overexpression of miR-483-5p and miR-483-3p and the concomitant down-regulation of miR-195 [55,56,57,58][55][56][57][58] and the combination of different altered miRNAs has been correlated with malignancy [55,56,59,60][55][56][59][60]. These miRNAs can thus be used to distinguish between benign and malignant adrenocortical tumors and are promising biomarkers with prognostic value in ACC patients [54,58,61][54][58][61].
Chromosomal alterations are also often present in ACC compared to ACA [62]. Previous analysis have shown specific amplifications in the chromosomal regions containing the TERT gene (5p15.33) and the CDK4 gene (12q14), and deletions in the chromosomal regions of the ZNRF3 (22q12.1), CDKN2A (9p21.3), and RB1 (13q14) genes [12,13,38][12][13][38]. Furthermore, genome analyses of ACC revealed frequent occurrence of massive DNA loss and loss of heterozygosity (LOH) followed by whole-genome doubling (WGD), which is associated with tumor aggressiveness, suggesting that WGD may represent a hallmark of disease progression [13].
Studies in the mutational landscape of ACC have allowed the identification of specific driver genes [12,13,38][12][13][38]. Among them, the most common altered gene is ZNRF3, which encodes an E3 ubiquitin ligase that negatively regulates the Wnt/beta-catenin pathway [12,13,38][12][13][38]. Other recurrently mutated genes are TP53, which is related to the cell cycle regulation, the tumor suppressor genes CDKN2A and RB1, oncogenes MDM2 and CDK4, and genes involved in chromatin remodeling (MEN1, DAXX, and ATRX) and chromatin maintenance (TERT and TERF2) [12,13,38][12][13][38]. Moreover, somatic mutations in genes involved in PKA activation, such as the PKA regulatory subunit PRKAR1A, have also been identified in ACC [13]. Proliferation and differentiation of the adrenocortical glucocorticoid-producing zona fasciculata is reliant on ACTH-dependent PKA signaling [63]. Additionally, cortisol-producing adenomas are characterized by abnormally high levels of PKA activation [53]. Furthermore, it was demonstrated that ACTH-dependent proliferation during zona fasciculata regeneration also relies on Wnt/β-catenin signaling [64]. This, associated with the identification of recurrent mutations leading to constitutive activation of both pathways in ACC [12,13][12][13], suggests that components of the ACTH signaling pathway may be implicated in the adrenocortical tumorigenesis. However, several genes involved in steroidogenesis are downregulated in ACC, when compared to ACA [65].
On the basis of these molecular features, it is possible to stratify ACC patients in three prognostic subgroups with different expected outcomes [11,13][11][13]. Therefore, the genomic profile allows a molecular classification of ACC and can be used to improve the diagnosis, prognosis, and management of patients with ACC, but also for the development of novel pharmacological strategies.
(References would be added automatically after the entry is online)
References
- Varghese, J.; Habra, M.A. Update on adrenocortical carcinoma management and future directions. Curr. Opin. Endocrinol. Diabetes Obes. 2017.
- Kerkhofs, T.M.A.; Verhoeven, R.H.A.; Van Der Zwan, J.M.; Dieleman, J.; Kerstens, M.N.; Links, T.P.; Van De Poll-Franse, L.V.; Haak, H.R. Adrenocortical carcinoma: A population-based study on incidence and survival in the Netherlands since 1993. Eur. J. Cancer 2013.
- Ayala-Ramirez, M.; Jasim, S.; Feng, L.; Ejaz, S.; Deniz, F.; Busaidy, N.; Waguespack, S.G.; Naing, A.; Sircar, K.; Wood, C.G.; et al. Adrenocortical carcinoma: Clinical outcomes and prognosis of 330 patients at a tertiary care Center. Eur. J. Endocrinol. 2013.
- Fassnacht, M.; Dekkers, O.M.; Else, T.; Baudin, E.; Berruti, A.; De Krijger, R.R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European society of endocrinology clinical practice guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the study of adrenal tumors. Eur. J. Endocrinol. 2018.
- Kerkhofs, T.M.; Baudin, E.; Terzolo, M.; Allolio, B.; Chadarevian, R.; Mueller, H.H.; Skogseid, B.; Leboulleux, S.; Mantero, F.; Haak, H.R.; et al. Comparison of two mitotane starting dose regimens in patients with advanced adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2013.
- Berruti, A.; Terzolo, M.; Sperone, P.; Pia, A.; Della Casa, S.; Gross, D.J.; Carnaghi, C.; Casali, P.; Porpiglia, F.; Mantero, F.; et al. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: A large prospective phase II trial. Endocr. Relat. Cancer 2005.
- Megerle, F.; Kroiss, M.; Hahner, S.; Fassnacht, M. Advanced Adrenocortical Carcinoma-What to do when First-Line Therapy Fails? Exp. Clin. Endocrinol. Diabetes 2019.
- Sinclair, T.J.; Gillis, A.; Alobuia, W.M.; Wild, H.; Kebebew, E. Surgery for adrenocortical carcinoma: When and how? Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101408.
- Fassnacht, M.; Terzolo, M.; Allolio, B.; Baudin, E.; Haak, H.; Berruti, A.; Welin, S.; Schade-Brittinger, C.; Lacroix, A.; Jarzab, B.; et al. Combination Chemotherapy in Advanced Adrenocortical Carcinoma. N. Engl. J. Med. 2012.
- Atallah, S.; Al-Assaf, H.; Xu, Y.; El-Sayed, S. Adrenocortical carcinoma: Patterns of care and role of adjuvant radiation therapy— a population-based study and review of the literature. Curr. Oncol. 2017.
- Armignacco, R.; Cantini, G.; Canu, L.; Poli, G.; Ercolino, T.; Mannelli, M.; Luconi, M. Adrenocortical carcinoma: The dawn of a new era of genomic and molecular biology analysis. J. Endocrinol. Investig. 2018.
- Assié, G.; Letouzé, E.; Fassnacht, M.; Jouinot, A.; Luscap, W.; Barreau, O.; Omeiri, H.; Rodriguez, S.; Perlemoine, K.; René-Corail, F.; et al. Integrated genomic characterization of adrenocortical carcinoma. Nat. Genet. 2014.
- Zheng, S.; Cherniack, A.D.; Dewal, N.; Moffitt, R.A.; Danilova, L.; Murray, B.A.; Lerario, A.M.; Else, T.; Knijnenburg, T.A.; Ciriello, G.; et al. Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell 2016.
- Tissier, F.; Cavard, C.; Groussin, L.; Perlemoine, K.; Fumey, G.; Hagneré, A.M.; René-Corail, F.; Jullian, E.; Gicquel, C.; Bertagna, X.; et al. Mutations of β-catenin in adrenocortical tumors: Activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005.
- Giordano, T.J.; Kuick, R.; Else, T.; Gauger, P.G.; Vinco, M.; Bauersfeld, J.; Sanders, D.; Thomas, D.G.; Doherty, G.; Hammer, G. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin. Cancer Res. 2009.
- Costa, R.; Carneiro, B.A.; Tavora, F.; Pai, S.G.; Kaplan, J.B.; Chae, Y.K.; Chandra, S.; Kopp, P.A.; Giles, F.J. The challenge of developmental therapeutics for adrenocortical carcinoma. Oncotarget 2016.
- De Martino, M.C.; Al Ghuzlan, A.; Aubert, S.; Assié, G.; Scoazec, J.Y.; Leboulleux, S.; Do Cao, C.; Libè, R.; Nozières, C.; Lombès, M.; et al. Molecular screening for a personalized treatment approach in advanced adrenocortical cancer. J. Clin. Endocrinol. Metab. 2013.
- Creemers, S.G.; Hofland, L.J.; Korpershoek, E.; Franssen, G.J.H.; Van Kemenade, F.J.; De Herder, W.W.; Feelders, R.A. Future directions in the diagnosis and medical treatment of adrenocortical carcinoma. Endocr. Relat. Cancer 2016.
- Konda, B.; Kirschner, L.S. Novel targeted therapies in adrenocortical carcinoma. Curr. Opin. Endocrinol. Diabetes Obes. 2016.
- Sperone, P.; Ferrero, A.; Daffara, F.; Priola, A.; Zaggia, B.; Volante, M.; Santini, D.; Vincenzi, B.; Badalamenti, G.; Intrivici, C.; et al. Gemcitabine plus metronomic 5-fluorouracil or capecitabine as a second-/third-line chemotherapy in advanced adrenocortical carcinoma: A multicenter phase II study. Endocr. Relat. Cancer 2010.
- Henning, J.E.K.; Deutschbein, T.; Altieri, B.; Steinhauer, S.; Kircher, S.; Sbiera, S.; Wild, V.; Schlötelburg, W.; Kroiss, M.; Perotti, P.; et al. Gemcitabine-based chemotherapy in adrenocortical carcinoma: A multicenter study of efficacy and predictive factors. J. Clin. Endocrinol. Metab. 2017.
- Khan, T.S.; Imam, H.; Juhlin, C.; Skogseid, B.; Gröndal, S.; Tibblin, S.; Wilander, E.; Öberg, K.; Eriksson, B. Streptozocin and o,p’DDD in the treatment of adrenocortical cancer patients: Long-term survival in its adjuvant use. Ann. Oncol. 2000.
- Berruti, A.; Sperone, P.; Ferrero, A.; Germano, A.; Ardito, A.; Priola, A.M.; De Francia, S.; Volante, M.; Daffara, F.; Generali, D.; et al. Phase II study of weekly paclitaxel and sorafenib as second/third-line therapy in patients with adrenocortical carcinoma. Eur. J. Endocrinol. 2012.
- De Martino, M.C.; Feelders, R.A.; Pivonello, C.; Simeoli, C.; Papa, F.; Colao, A.; Pivonello, R.; Hofland, L.J. The role of mTOR pathway as target for treatment in adrenocortical cancer. Endocr. Connect. 2019, 8, R144–R156.
- Fassnacht, M.; Berruti, A.; Baudin, E.; Demeure, M.J.; Gilbert, J.; Haak, H.; Kroiss, M.; Quinn, D.I.; Hesseltine, E.; Ronchi, C.L.; et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: A double-blind, randomised, phase 3 study. Lancet Oncol. 2015.
- De Martino, M.C.; van Koetsveld, P.M.; Feelders, R.A.; de Herder, W.W.; Dogan, F.; Janssen, J.A.M.J.L.; Hofste op Bruinink, D.; Pivonello, C.; Waaijers, A.M.; Colao, A.; et al. IGF and mTOR pathway expression and in vitro effects of linsitinib and mTOR inhibitors in adrenocortical cancer. Endocrine 2019.
- Le Tourneau, C.; Hoimes, C.; Zarwan, C.; Wong, D.J.; Bauer, S.; Claus, R.; Wermke, M.; Hariharan, S.; Von Heydebreck, A.; Kasturi, V.; et al. Avelumab in patients with previously treated metastatic adrenocortical carcinoma: Phase 1b results from the JAVELIN solid tumor trial. J. Immunother. Cancer 2018.
- Carneiro, B.A.; Konda, B.; Costa, R.B.; Costa, R.L.B.; Sagar, V.; Gursel, D.B.; Kirschner, L.S.; Chae, Y.K.; Abdulkadir, S.A.; Rademaker, A.; et al. Nivolumab in Metastatic Adrenocortical Carcinoma: Results of a Phase 2 Trial. J. Clin. Endocrinol. Metab. 2019.
- Habra, M.A.; Stephen, B.; Campbell, M.; Hess, K.; Tapia, C.; Xu, M.; Rodon Ahnert, J.; Jimenez, C.; Lee, J.E.; Perrier, N.D.; et al. Phase II clinical trial of pembrolizumab efficacy and safety in advanced adrenocortical carcinoma. J. Immunother. Cancer 2019.
- Raj, N.; Zheng, Y.; Kelly, V.; Katz, S.S.; Chou, J.; Do, R.K.G.; Capanu, M.; Zamarin, D.; Saltz, L.B.; Ariyan, C.E.; et al. PD-1 blockade in advanced adrenocortical carcinoma. J. Clin. Oncol. 2020.
- McGregor, B.A.; Campbell, M.T.; Xie, W.; Farah, S.; Bilen, M.A.; Schmidt, A.L.; Sonpavde, G.P.; Kilbridge, K.L.; Choudhury, A.D.; Mortazavi, A.; et al. Results of a multicenter, phase 2 study of nivolumab and ipilimumab for patients with advanced rare genitourinary malignancies. Cancer 2020, 1–10.
- Billon, E.; Finetti, P.; Bertucci, A.; Niccoli, P.; Birnbaum, D.; Mamessier, E.; Bertucci, F. PDL1 expression is associated with longer postoperative, survival in adrenocortical carcinoma. Oncoimmunology 2019.
- Raymond, V.M.; Everett, J.N.; Furtado, L.V.; Gustafson, S.L.; Jungbluth, C.R.; Gruber, S.B.; Hammer, G.D.; Stoffel, E.M.; Greenson, J.K.; Giordano, T.J.; et al. Adrenocortical carcinoma is a lynch syndrome-associated cancer. J. Clin. Oncol. 2013, 31, 3012.
- Gara, S.K.; Lack, J.; Zhang, L.; Harris, E.; Cam, M.; Kebebew, E. Metastatic adrenocortical carcinoma displays higher mutation rate and tumor heterogeneity than primary tumors. Nat. Commun. 2018.
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA approval summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin. Cancer Res. 2019.
- Cosentini, D.; Grisanti, S.; Volta, A.D.; Laganà, M.; Fiorentini, C.; Perotti, P.; Sigala, S.; Berruti, A. Immunotherapy failure in adrenocortical cancer: Where next? Endocr. Connect. 2018.
- Fiorentini, C.; Grisanti, S.; Cosentini, D.; Abate, A.; Rossini, E.; Berruti, A.; Sigala, S. Molecular Drivers of Potential Immunotherapy Failure in Adrenocortical Carcinoma. J. Oncol. 2019.
- Juhlin, C.C.; Goh, G.; Healy, J.M.; Fonseca, A.L.; Scholl, U.I.; Stenman, A.; Kunstman, J.W.; Brown, T.C.; Overton, J.D.; Mane, S.M.; et al. Whole-exome sequencing characterizes the landscape of somatic mutations and copy number alterations in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2015.
- Assié, G.; Guillaud-Bataille, M.; Ragazzon, B.; Bertagna, X.; Bertherat, J.; Clauser, E. The pathophysiology, diagnosis and prognosis of adrenocortical tumors revisited by transcriptome analyses. Trends Endocrinol. Metab. 2010.
- Giordano, T.J.; Thomas, D.G.; Kuick, R.; Lizyness, M.; Misek, D.E.; Smith, A.L.; Sanders, D.; Aljundi, R.T.; Gauger, P.G.; Thompson, N.W.; et al. Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am. J. Pathol. 2003.
- De Fraipont, F.; El Atifi, M.; Cherradi, N.; Le Moigne, G.; Defaye, G.; Houlgatte, R.; Bertherat, J.; Bertagna, X.; Plouin, P.F.; Baudin, E.; et al. Gene expression profiling of human adrenocortical tumors using complementary deoxyribonucleic acid microarrays identifies several candidate genes as markers of malignancy. J. Clin. Endocrinol. Metab. 2005.
- Heaton, J.H.; Wood, M.A.; Kim, A.C.; Lima, L.O.; Barlaskar, F.M.; Almeida, M.Q.; Fragoso, M.C.B.V.; Kuick, R.; Lerario, A.M.; Simon, D.P.; et al. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and β-catenin. Am. J. Pathol. 2012.
- Ragazzon, B.; Libé, R.; Gaujoux, S.; Assié, G.; Fratticci, A.; Launay, P.; Clauser, E.; Bertagna, X.; Tissier, F.; De Reyniès, A.; et al. Transcriptome analysis reveals that p53 and β-catenin alterations occur in a group of aggressive adrenocortical cancers. Cancer Res. 2010.
- De Reyniès, A.; Assié, G.; Rickman, D.S.; Tissier, F.; Groussin, L.; René-Corail, F.; Dousset, B.; Bertagna, X.; Clauser, E.; Bertherat, J. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J. Clin. Oncol. 2009.
- Kulis, M.; Esteller, M. DNA Methylation and Cancer. Adv. Genet. 2010, 70, 27–56.
- Kalari, S.; Pfeifer, G.P. Identification of Driver and Passenger DNA Methylation in Cancer by Epigenomic Analysis. Adv. Genet. 2010, 70, 277–308.
- Rechache, N.S.; Wang, Y.; Stevenson, H.S.; Killian, J.K.; Edelman, D.C.; Merino, M.; Zhang, L.; Nilubol, N.; Stratakis, C.A.; Meltzer, P.S.; et al. DNA methylation profiling identifies global methylation differences and markers of adrenocortical tumors. J. Clin. Endocrinol. Metab. 2012.
- Fonseca, A.L.; Kugelberg, J.; Starker, L.F.; Scholl, U.; Choi, M.; Hellman, P.; Åkerström, G.; Westin, G.; Lifton, R.P.; Björklund, P.; et al. Comprehensive DNA methylation analysis of benign and malignant adrenocortical tumors. Genes Chromosom. Cancer 2012.
- Barreau, O.; Assié, G.; Wilmot-Roussel, H.; Ragazzon, B.; Baudry, C.; Perlemoine, K.; René-Corail, F.; Bertagna, X.; Dousset, B.; Hamzaoui, N.; et al. Identification of a CpG island methylator phenotype in adrenocortical carcinomas. J. Clin. Endocrinol. Metab. 2013.
- Jouinot, A.; Assie, G.; Libe, R.; Fassnacht, M.; Papathomas, T.; Barreau, O.; De La Villeon, B.; Faillot, S.; Hamzaoui, N.; Neou, M.; et al. DNA methylation is an independent prognostic marker of survival in adrenocortical cancer. J. Clin. Endocrinol. Metab. 2017.
- Creemers, S.G.; Van Koetsveld, P.M.; Van Kemenade, F.J.; Papathomas, T.G.; Franssen, G.J.H.; Dogan, F.; Eekhoff, E.M.W.; Van Der Valk, P.; De Herder, W.W.; Janssen, J.A.M.J.L.; et al. Methylation of IGF2 regulatory regions to diagnose adrenocortical carcinomas. Endocr. Relat. Cancer 2016.
- Cherradi, N. MicroRNAs as potential biomarkers in adrenocortical cancer: Progress and challenges. Front. Endocrinol. 2016.
- Lerario, A.M.; Moraitis, A.; Hammer, G.D. Genetics and epigenetics of adrenocortical tumors. Mol. Cell. Endocrinol. 2014.
- Igaz, P.; Igaz, I.; Nagy, Z.; Nyíro, G.; Szabó, P.M.; Falus, A.; Patócs, A.; Rácz, K. MicroRNAs in adrenal tumors: Relevance for pathogenesis, diagnosis, and therapy. Cell. Mol. Life Sci. 2015.
- Soon, P.S.H.; Tacon, L.J.; Gill, A.J.; Bambach, C.P.; Sywak, M.S.; Campbell, P.R.; Yeh, M.W.; Wong, S.G.; Clifton-Bligh, R.J.; Robinson, B.G.; et al. miR-195 and miR-483-5p identified as predictors of poor prognosis in adrenocortical cancer. Clin. Cancer Res. 2009.
- Patterson, E.E.; Holloway, A.K.; Weng, J.; Fojo, T.; Kebebew, E. MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer 2011.
- Özata, D.M.; Caramuta, S.; Velázquez-Fernández, D.; Akçakaya, P.; Xie, H.; Höög, A.; Zedenius, J.; Bäckdahl, M.; Larsson, C.; Lui, W.O. The role of microRNA deregulation in the pathogenesis of adrenocortical carcinoma. Endocr. Relat. Cancer 2011.
- Chabre, O.; Libé, R.; Assie, G.; Barreau, O.; Bertherat, J.; Bertagna, X.; Feige, J.J.; Cherradi, N. Serum miR-483-5p and miR-195 are predictive of recurrence risk in adrenocortical cancer patients. Endocr. Relat. Cancer 2013.
- Tömböl, Z.; Szabó, P.M.; Molnár, V.; Wiener, Z.; Tölgyesi, G.; Horányi, J.; Riesz, P.; Reismann, P.; Patócs, A.; Likó, I.; et al. Integrative molecular bioinformatics study of human adrenocortical tumors: MicroRNA, tissue-specific target prediction, and pathway analysis. Endocr. Relat. Cancer 2009.
- Schmitz, K.J.; Helwig, J.; Bertram, S.; Sheu, S.Y.; Suttorp, A.C.; Seggewiß, J.; Willscher, E.; Walz, M.K.; Worm, K.; Schmid, K.W. Differential expression of microRNA-675, microRNA-139-3p and microRNA-335 in benign and malignant adrenocortical tumours. J. Clin. Pathol. 2011.
- Szabó, D.R.; Luconi, M.; Szabó, P.M.; Tóth, M.; Szücs, N.; Horányi, J.; Nagy, Z.; Mannelli, M.; Patócs, A.; Rácz, K.; et al. Analysis of circulating microRNAs in adrenocortical tumors. Lab. Investig. 2014.
- Assié, G.; Jouinot, A.; Bertherat, J. The “omics” of adrenocortical tumours for personalized medicine. Nat. Rev. Endocrinol. 2014.
- Xing, Y.; Lerario, A.M.; Rainey, W.; Hammer, G.D. Development of Adrenal Cortex Zonation. Endocrinol. Metab. Clin. N. Am. 2015.
- Finco, I.; Lerario, A.M.; Hammer, G.D. Sonic hedgehog and WNT signaling promote adrenal gland regeneration in male mice. Endocrinology 2018.
- Ragazzon, B.; Assié, G.; Bertherat, J. Transcriptome analysis of adrenocortical cancers: From molecular classification to the identification of new treatments. Endocr. Relat. Cancer 2011.
