The SLC25A20 transporter, also known as carnitine acyl-carnitine carrier (CAC), catalyzes the transport of short, medium and long carbon chain acyl-carnitines across the mitochondrial inner membrane in exchange for carnitine. The 30-year story of the protein responsible for this function started with its purification from rat liver mitochondria.
- carnitine
- carnitine acyl-carnitine carrier
- carnitine acyl-carnitine translocase
- membrane transport
- mitochondria
- mitochondrial carrier
1. Introduction
The mitochondrial carnitine acyl-carnitine carrier (CAC) is the member A20 of the SLC25 protein family, including 53 solute transporters in humans, the majority of which are localized in the inner mitochondrial membrane. Until now, only one family member has been found in the peroxisomal membrane. Furthermore, approximately one-third of them are still orphans, i.e., their transported substrates are unknown. This family members share a peculiar structural fold of six transmembrane segments characterized by 3-fold repeated couples of hydrophobic α-helices. Each couple is connected by a hydrophilic loop and contains the SLC25 sequence motif PX[D/E]XX[K/R] at about the boundary of the odd α-helix and the loop. The structural information on the SLC25 proteins derives mainly from the ADP/ATP carrier, which has been crystallized in both the outwards and inward open conformations. All the other carrier structures have been predicted by homology modeling, including CAC, whose structure has been corroborated by site-directed mutagenesis and chemical targeting approaches. CAC is a key component of the carnitine shuttle, which is crucial for the mitochondrial β-oxidation pathway. In this shuttle (Figure 1), fatty acids are activated by the cytosolic acyl-CoA synthetase (ACSL) to fatty acyl-CoAs thioesters. Since the mitochondrial inner membrane is not permeable to acyl-CoAs, acyl groups are transferred from CoA to carnitine by the action of “carnitine palmitoyltransferase-1a and b” (CPT-1a; CPT-1b), an integral outer membrane enzyme. The acyl-carnitines cross the outer mitochondrial membrane through an almost unspecific pore constituted by the voltage-dependent anion channel (VDAC) and, then, are specifically translocated across the inner mitochondrial membrane by the action of CAC. In the mitochondrial matrix, the enzyme carnitine palmitoyltransferase 2 (CPT-2) catalyzes the trans-esterification of the acyl groups from carnitine to mitochondrial CoA with the release of free carnitine, thereby providing acyl-CoA substrates for fatty acid β-oxidation. CAC and CPT-2 form a supramolecular complex in the inner mitochondrial membrane, devoted to acyl-carnitine channeling from the carrier to the enzyme (Figure 1). The carnitine released in this reaction is translocated backward to the cytosol by the same carrier via an acyl-carnitine/carnitine antiport reaction. The β-oxidation pathway is active in many tissues, especially those characterized by higher metabolic expenditure. It provides a large portion of the energy required by heart muscle, kidneys and also skeletal muscle, when glycogen has been consumed. This pathway is also active in hepatocytes where fatty acid oxidation provides acetyl-CoA for ketone body synthesis during prolonged fasting conditions, in which glycogen stores have been depleted. Neurons also perform fatty acid oxidation even though at a very low rate. Indeed, CAC also has been described in brain. The crucial role of CAC in energy metabolism was demonstrated by the discovery of inherited defects of its gene SLC25A20 causing secondary carnitine deficiency, a syndrome that arises in the very first stage of life as a life-threatening pathology. In this altered metabolic condition, acyl-carnitines fail to reach the mitochondrial matrix with consequent strong impairment of the β-oxidation. This syndrome is more severe than the primary carnitine deficiency caused by defects of the plasma membrane transporter OCTN2 (SLC22A5). Recent findings have correlated alterations of CAC expression or regulation with diabetes.
Unlike most mitochondrial carriers, which are obligatory antiporters, CAC can catalyze, besides the antiport reaction, also a unidirectional transport of substrates event though at a rate about one order of magnitude lower than the antiport. Interestingly CAC is not only operating in animals but also yeast and plants. The Saccharomyces cerevisiae and the Aspergillus nidulans CACs share 29% and 42% identity with the human CAC, respectively. The main function of these transporters, in contrast to that of mammalian CACs, is to transport acetylcarnitine rather than medium- and long-chain acyl-carnitines into mitochondria. The plant CAC ortholog, identified based on the 37% sequence identity with the human counterpart, most probably plays a different role, that is, the transport of glutamate. It is still not clear if CAC also operates in peroxisomes, where very long, branched-chain, and medium-chain fatty acids are imported.
The history of CAC started with the detection of an acyl-carnitine uptake into mitochondria, which was saturable, stereospecific, inhibitable, and temperature-dependent. Then, the availability of methodologies capable of handling hydrophobic membrane proteins allowed us to purify the protein responsible for the observed transport phenomena. In 1990, a classical approach based on chromatography fractionation of a rat liver mitochondrial extract and on transport assay of the fractions by proteoliposome technology was adopted. The purified protein was used for the first functional characterization. Later, CAC was identified at a molecular level and obtained on a large-scale by overexpression in Escherichia coli by a procedure introduced in our laboratory for the bacterial overexpression of the oxoglutarate carrier and recently named the expression, purification, reconstitution assay (EPRA) method. The recombinant purified CAC was employed in studies of structure/function relationships, interaction with drugs and xenobiotics, and post-translational modifications that modulate its transport function.
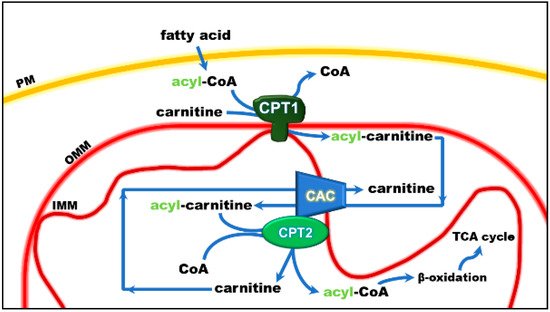
Figure 1. Role of the carnitine shuttle in the mitochondrial β-oxidation pathway. The shuttle is constituted by carnitine palmitoyltransferase 1 (CPT1) that converts acyl-CoAs into acyl-carnitines; carnitine/acyl-carnitine carrier (CAC) that allows the uptake of acyl-carnitines in the mitochondrial matrix in exchange with free carnitine, and carnitine palmitoyltransferase 2 (CPT2) that converts acyl-carnitines back to acyl-CoAs and releases free carnitine, which is ready to be translocated back to the cytosol by CAC. Once in the matrix, acyl-CoA undergoes β-oxidation with the production of acetyl-CoA that enters the tricarboxylic acid cycle (TCA). Other abbreviations: IMM, inner mitochondrial membrane; OMM, outer mitochondrial membrane; PM, plasma membrane.
2. The Functional Role of CAC
Studies performed with intact mitochondria concurred to propose that the function of CAC in cells is that of catalyzing an antiport of acyl-carnitines with free carnitine according to the core activity of the carnitine shuttle [1] (Figure 1). Physiologically, the acyl-carnitines are transported from the cytosol to the mitochondrial matrix and the free carnitine in the opposite direction to sustain the intramitochondrial reactions of the β-oxidation pathway [2][3]. Later on, the studies in proteoliposomes confirmed this function. In the in vitro system, CAC purified from rat liver or recombinant CAC was inserted into the liposomal membrane with the same orientation as in the native membrane, thus representing a mitochondrion mimic single-protein model [4][5] (Figure 2).
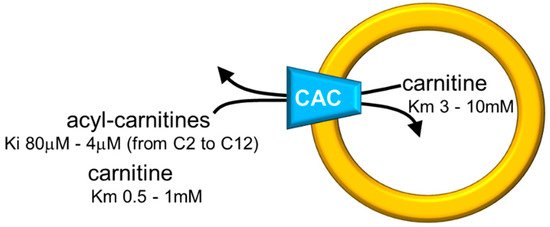
Figure 2. Sketch of the proteoliposome model with reconstituted CAC. Proteoliposomes contained 4% cardiolipin. Range of inhibition constants (Ki) for acyl-carnitines of various chain lengths on the external side and ranges of carnitine Km on both sides of CAC are reported.
The in vitro system allowed measurements of substrate affinity (Figure 2), giving further support to the preferential direction of transport of acyl-carnitines towards the internal space of proteoliposomes corresponding to the mitochondrial matrix and carnitine in the counter-direction. In vivo, this transport mode is driven by the higher acyl-carnitine concentration (see Figure 1) in the cytosol than in the matrix space, where acyl-carnitines are rapidly removed by the action of CPT2 that strictly interacts with the carrier [6][7]. Interestingly, the affinity profile of the carrier follows the specificity for acyl-carnitines of the CPT1 [8][9]. The possibility to manage the single-protein experimental system led to establishing that CAC catalyzes the transport of acetyl-carnitine too [10], suggesting that shorter carbon chain esters of carnitine across the inner mitochondrial membrane via CAC as the longer chain derivatives, in contrast to what previously hypothesized [11]. This indicates the role of CAC in participating in the scavenger action of acetyl-CoA from mitochondria [5][12][13]. In this frame, the matrix enzyme carnitine acetyltransferase converts acetyl-CoA to acetyl-carnitine, which can be exported from mitochondria in antiport with extramitochondrial carnitine. In this pathway, CAC works in a reverse mode mediating the efflux of carnitine derivatives from mitochondria [14]. However, a definitive demonstration of the reverse mode of action in vivo is still missing. The affinity of CAC for acetyl-carnitine is much lower than that for long-chain acyl-carnitines, at least on the external face of the carrier (Figure 2). Using the in vitro experimental system, a bisubstrate kinetic study carried out by varying both the internal and the external substrate concentrations demonstrated that CAC catalyzes the antiport of substrates according to a “ping-pong mechanism” [15][4][16]. This mechanism of transport involves only binary carrier substrate complexes and implies that CAC possesses a single “reorienting” binding site and two conformations, one with the substrate-binding site accessible from the cytosol and the other with the substrate-binding site accessible from the matrix. Therefore, the ping-pong mechanism, so named for the analogy with that of certain enzymes, is basically the same mechanism as the early hypothesized “single binding center-gating pore mechanism” [17][18] and as the recently described “alternating access mechanism” [19], which is based on numerous molecular details. It is worth mentioning that, according to the ping-pong mechanism, the Km for carnitine on the external or the internal side of CAC is influenced by the counter-substrate concentration, thus being variable within a certain range (Figure 2).
CAC also catalyzes a uniport reaction with a lower rate compared to the antiport. This almost unique feature among mitochondrial carriers was known since the 80s from studies in intact mitochondria [20] and later was confirmed by studies performed with proteoliposomes [21][4]. The rate of the unidirectional transport of carnitine is regulated by the counter-substrate; the uniport progressively decreases by increasing the concentration of the counter-substrate until the antiport mode is triggered. Physiologically, the net flux of carnitine allows for providing the matrix with carnitine newly synthesized in the cytosol or absorbed from the diet [21]. It must be stressed that (i) the last step of the carnitine biosynthesis occurs in the cytosol where the enzyme γ-butyrobetaine dioxygenase is located [22][23], (ii) the endogenous synthesis is not sufficient for the body’s needs, and (iii) more than 50% of carnitine is absorbed from the diet [24][25][26][27]. Therefore, the entire mitochondrial carnitine pool derives from extramitochondrial sources. As said before, CAC provides the matrix with carnitine through the uniport function. This role is crucial during mitochondrial biogenesis; however, no information is available on this issue. The uniport function should also be important for net export of carnitine to allow carnitine excretion and renewal. Very little information is available on the carnitine recycling. Indeed, even though it is known that an aliquot of carnitine is excreted through the urine, the flux of the molecule from the mitochondrial matrix to be excreted has never been dealt with.
Early studies showed the high sensitivity of CAC to sulfhydryl reagents [3][28]. Subsequent studies in proteoliposomes led to discrimination between two functional alterations caused by the reaction of SH reagents with two different cysteine populations: class-I cysteines are responsible for the induction of an “unphysiological” unspecific uniport; class-II cysteines are responsible for the inactivation of the carrier (both antiport and uniport function). On the one hand, the reaction of class-I cysteines with HgCl2 or mercurial derivatives, at relatively high concentration, converts the carrier to a “pore-like” transporter with reduced substrate specificity and uncoupling of the antiport function. This unphysiological activity reveals an intrinsic property of the mitochondrial carrier protein family members, i.e., a built-in channel normally hidden by appropriate gates [29][30]. Indeed, this phenomenon also has been observed with other mitochondrial carrier proteins [31][32][33]. On the other hand, the reaction of class-II cysteines with HgCl2 and other mercurials at a low (nanomolar) concentration or with NEM and MTS leads to the inactivation of the transporter. Later, class-II Cys residues and the molecular basis of their inhibition were identified. This aspect will be dealt with in the following sections. In contrast, class-I Cys residues responsible for pore-like activity have not yet been identified.
Overexpression of recombinant CAC in E. coli [5] boosted the characterization of this transporter. Indeed, the recombinant protein showed the same properties as the native one indicating that it is suitable for functional studies. This breakthrough opened the perspective of studying the human CAC as well [34][35]. Novel functional information was achieved in a rather short time. The absolute need for cardiolipin, suggested by studies with the protein purified from rat liver, was clearly demonstrated with the recombinant CAC that is cardiolipin free and is inactive if not supplemented with the phospholipid [5][36][37]. Therefore, CAC belongs to the mitochondrial molecular systems, which require cardiolipin for an activity like many other mitochondrial carriers [38][39][40][41][42] or are modulated by cardiolipin as the NADH dehydrogenase [43][44]. Other important achievements following the involvement of recombinant CAC and site-directed mutagenesis strategy will be dealt with in the next section.
References
- Stanley, C.A.; Palmieri, F.; Bennett, M.J. Disorders of the mitochondrial carnitine shuttle. Online Metab. Mol. Bases Inherit. Dis. 2013.
- Pande, S.V. A mitochondrial carnitine acylcarnitine translocase system. Proc. Natl. Acad. Sci. USA 1975, 72, 883–887.
- Murthy, M.S.; Pande, S.V. Mechanism of carnitine acylcarnitine translocase-catalyzed import of acylcarnitines into mitochondria. J. Biol. Chem. 1984, 259, 9082–9089.
- Indiveri, C.; Tonazzi, A.; Palmieri, F. The reconstituted carnitine carrier from rat liver mitochondria: Evidence for a transport mechanism different from that of the other mitochondrial translocators. Biochim. Biophys. Acta 1994, 1189, 65–73.
- Indiveri, C.; Iacobazzi, V.; Giangregorio, N.; Palmieri, F. Bacterial overexpression, purification, and reconstitution of the carnitine/acylcarnitine carrier from rat liver mitochondria. Biochem. Biophys. Res. Commun. 1998, 249, 589–594.
- Indiveri, C.; Tonazzi, A.; Prezioso, G.; Palmieri, F. Kinetic characterization of the reconstituted carnitine carrier from rat liver mitochondria. Biochim. Biophys. Acta 1991, 1065, 231–238.
- Tonazzi, A.; Console, L.; Giangregorio, N.; Indiveri, C.; Palmieri, F. Identification by site-directed mutagenesis of a hydrophobic binding site of the mitochondrial carnitine/acylcarnitine carrier involved in the interaction with acyl groups. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 697–704.
- Bartelds, B.; Takens, J.; Smid, G.B.; Zammit, V.A.; Prip-Buus, C.; Kuipers, J.R.; van der Leij, F.R. Myocardial carnitine palmitoyltransferase I expression and long-chain fatty acid oxidation in fetal and newborn lambs. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2243–H2248.
- Eaton, S. Control of mitochondrial beta-oxidation flux. Prog. Lipid Res. 2002, 41, 197–239.
- Scalise, M.; Galluccio, M.; Pochini, L.; Console, L.; Barile, M.; Giangregorio, N.; Tonazzi, A.; Indiveri, C. Studying Interactions of Drugs with Cell Membrane Nutrient Transporters: New Frontiers of Proteoliposome Nanotechnology. Curr. Pharm. Des. 2017, 23, 3871–3883.
- Violante, S.; Ijlst, L.; Te Brinke, H.; Tavares de Almeida, I.; Wanders, R.J.; Ventura, F.V.; Houten, S.M. Carnitine palmitoyltransferase 2 and carnitine/acylcarnitine translocase are involved in the mitochondrial synthesis and export of acylcarnitines. FASEB J. 2013, 27, 2039–2044.
- Arduini, A.; Zammit, V. Acetate transport into mitochondria does not require a carnitine shuttle mechanism. Magn. Reson. Med. 2017, 77, 11.
- Ramsay, R.; Arduini, A. The carnitine acyltransferases and their role in modulating acyl-CoA pools. Arch. Biochem. Biophys. 1993, 302, 307–314.
- Davies, M.N.; Kjalarsdottir, L.; Thompson, J.W.; Dubois, L.G.; Stevens, R.D.; Ilkayeva, O.R.; Brosnan, M.J.; Rolph, T.P.; Grimsrud, P.A.; Muoio, D.M. The Acetyl Group Buffering Action of Carnitine Acetyltransferase Offsets Macronutrient-Induced Lysine Acetylation of Mitochondrial Proteins. Cell Rep. 2016, 14, 243–254.
- Palmieri, F. The mitochondrial transporter family SLC25: Identification, properties and physiopathology. Mol. Aspects Med. 2013, 34, 465–484.
- Bisaccia, F.; De Palma, A.; Dierks, T.; Krämer, R.; Palmieri, F. Reaction mechanism of the reconstituted tricarboxylate carrier from rat liver mitochondria. Biochim. Biophys. Acta 1993, 1142, 139–145.
- Klingenberg, M. The ADP, ATP shuttle of the mitochondrion. Trends Biochem. Sci. 1979, 4, 249–252.
- Klingenberg, M. The ADP and ATP transport in mitochondria and its carrier. Biochim. Biophys. Acta 2008, 1778, 1978–2021.
- Ruprecht, J.J.; King, M.S.; Zögg, T.; Aleksandrova, A.A.; Pardon, E.; Crichton, P.G.; Steyaert, J.; Kunji, E.R.S. The Molecular Mechanism of Transport by the Mitochondrial ADP/ATP Carrier. Cell 2019, 176, 435–447.e415.
- Pande, S.V.; Parvin, R. Carnitine-acylcarnitine translocase catalyzes an equilibrating unidirectional transport as well. J. Biol. Chem. 1980, 255, 2994–3001.
- Indiveri, C.; Tonazzi, A.; Palmieri, F. Characterization of the unidirectional transport of carnitine catalyzed by the reconstituted carnitine carrier from rat liver mitochondria. Biochim. Biophys. Acta 1991, 1069, 110–116.
- Longo, N.; Amat di San Filippo, C.; Pasquali, M. Disorders of carnitine transport and the carnitine cycle. Am. J. Med. Genet. C Semin Med. Genet. 2006, 142C, 77–85.
- Vaz, F.M.; Wanders, R.J. Carnitine biosynthesis in mammals. Biochem. J. 2002, 361, 417–429.
- Pochini, L.; Galluccio, M.; Scalise, M.; Console, L.; Indiveri, C. OCTN: A Small Transporter Subfamily with Great Relevance to Human Pathophysiology, Drug Discovery, and Diagnostics. SLAS Discov. 2019, 24, 89–110.
- Noland, R.C.; Koves, T.R.; Seiler, S.E.; Lum, H.; Lust, R.M.; Ilkayeva, O.; Stevens, R.D.; Hegardt, F.G.; Muoio, D.M. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J. Biol. Chem. 2009, 284, 22840–22852.
- Almannai, M.; Alfadhel, M.; El-Hattab, A.W. Carnitine Inborn Errors of Metabolism. Molecules 2019, 24, 3251.
- El-Hattab, A.W.; Scaglia, F. Disorders of carnitine biosynthesis and transport. Mol. Genet. Metab. 2015, 116, 107–112.
- Pande, S.V.; Parvin, R. Characterization of carnitine acylcarnitine translocase system of heart mitochondria. J. Biol. Chem. 1976, 251, 6683–6691.
- Indiveri, C.; Tonazzi, A.; Dierks, T.; Krämer, R.; Palmieri, F. The mitochondrial carnitine carrier: Characterization of SH-groups relevant for its transport function. Biochim. Biophys. Acta 1992, 1140, 53–58.
- Indiveri, C.; Tonazzi, A.; Giangregorio, N.; Palmieri, F. Probing the active site of the reconstituted carnitine carrier from rat liver mitochondria with sulfhydryl reagents. A cysteine residue is localized in or near the substrate binding site. Eur. J. Biochem. 1995, 228, 271–278.
- Tonazzi, A.; Indiveri, C. Chemical modification of the mitochondrial ornithine/citrulline carrier by SH reagents: Effects on the transport activity and transition from carrier to pore-like function. Biochim. Biophys. Acta Biomembr. 2003, 1611, 123–130.
- Krämer, R. Mitochondrial carrier proteins can reversibly change their transport mode: The cases of the aspartate/glutamate and the phosphate carrier. Exp. Physiol. 1998, 83, 259–265.
- Stappen, R.; Kramer, R. Functional properties of the reconstituted phosphate carrier from bovine heart mitochondria: Evidence for asymmetric orientation and characterization of three different transport modes. Biochim. Biophys. Acta 1993, 1149, 40–48.
- De Lucas, J.R.; Indiveri, C.; Tonazzi, A.; Perez, P.; Giangregorio, N.; Iacobazzi, V.; Palmieri, F. Functional characterization of residues within the carnitine/acylcarnitine translocase RX(2)PANAAXF distinct motif. Mol. Membr. Biol. 2008, 25, 152–163.
- Giangregorio, N.; Tonazzi, A.; Console, L.; Indiveri, C.; Palmieri, F. Site-directed mutagenesis of charged amino acids of the human mitochondrial carnitine/acylcarnitine carrier: Insight into the molecular mechanism of transport. Biochim. Biophys. Acta Biomembr. 2010, 1797, 839–845.
- Indiveri, C.; Giangregorio, N.; Iacobazzi, V.; Palmieri, F. Site-directed mutagenesis and chemical modification of the six native cysteine residues of the rat mitochondrial carnitine carrier: Implications for the role of cysteine-136. Biochemistry 2002, 41, 8649–8656.
- Giangregorio, N.; Tonazzi, A.; Indiveri, C.; Palmieri, F. Conformation-dependent accessibility of Cys-136 and Cys-155 of the mitochondrial rat carnitine/acylcarnitine carrier to membrane-impermeable SH reagents. Biochim. Biophys. Acta Biomembr. 2007, 1767, 1331–1339.
- Agrimi, G.; Russo, A.; Scarcia, P.; Palmieri, F. The human gene SLC25A17 encodes a peroxisomal transporter of coenzyme A, FAD and NAD+. Biochem. J. 2012, 443, 241–247.
- Fiermonte, G.; Dolce, V.; Palmieri, F. Expression in Escherichia coli, functional characterization, and tissue distribution of isoforms A and B of the phosphate carrier from bovine mitochondria. J. Biol. Chem. 1998, 273, 22782–22787.
- Fiermonte, G.; Paradies, E.; Todisco, S.; Marobbio, C.M.; Palmieri, F. A novel member of solute carrier family 25 (SLC25A42) is a transporter of coenzyme A and adenosine 3′,5′-diphosphate in human mitochondria. J. Biol. Chem. 2009, 284, 18152–18159.
- Porcelli, V.; Fiermonte, G.; Longo, A.; Palmieri, F. The human gene SLC25A29, of solute carrier family 25, encodes a mitochondrial transporter of basic amino acids. J. Biol. Chem. 2014, 289, 13374–13384.
- Di Noia, M.A.; Todisco, S.; Cirigliano, A.; Rinaldi, T.; Agrimi, G.; Iacobazzi, V.; Palmieri, F. The human SLC25A33 and SLC25A36 genes of solute carrier family 25 encode two mitochondrial pyrimidine nucleotide transporters. J. Biol. Chem. 2014, 289, 33137–33148.
- Jussupow, A.; Di Luca, A.; Kaila, V.R.I. How cardiolipin modulates the dynamics of respiratory complex I. Sci. Adv. 2019, 5, eaav1850.
- Klingenberg, M. Cardiolipin and mitochondrial carriers. Biochim. Biophys. Acta 2009, 1788, 2048–2058.
