Alzheimer’s disease (AD) is a growing concern in modern society, and effective drugs for its treatment are lacking.
Uncaria rhynchophylla
(UR) and its main alkaloids have been studied to treat neurodegenerative diseases such as AD. This study aimed to uncover the key components and mechanism of the anti-AD effect of UR alkaloids through a network pharmacology approach.
- Alzheimer’s disease
- Uncaria rhynchophylla
- alkaloids
- network pharmacology
- AD pathology
1. Introduction
Alzheimer’s disease (AD), the most common form of dementia in the elderly, is characterized by the progressive deterioration of cognitive function and memory [1]. Approximately 50 million people were affected by AD worldwide in 2019, and nearly 10 million new cases occur every year [2]. The Chinese Center for Disease Control and Prevention estimated that there are more than 9 million AD patients in China at present, and it is expected that 40 million could be affected by 2050. AD not only brings a heavy mental burden and economic pressure to the family members and friends of patients but also places a very large burden on the social economy [3]. At present, the typical events in AD pathogenesis are considered to be the formation of extracellular amyloid-β (Aβ) plaques, intracellular accumulation of abnormally phosphorylated tau, neuronal synaptic dysfunction, and neuronal loss [4,5,6,7]. However, over the last decade, all clinical trials targeting a single target, such as Aβ or tau, have failed, and there are also no effective drugs for the prevention or treatment of AD in the clinic [8]. The anti-AD drugs currently approved by the Food and Drug Administration are cholinesterase inhibitors: donepezil, rivastigmine, tacrine, galantamine, and the N-methyl D-aspartate (NMDA) receptor antagonist memantine [9]. These drugs are symptomatic treatments and cannot halt the disease course. Moreover, the above drugs also cause a variety of unfavorable effects, including hypertensive crisis, nausea, diarrhea, and vomiting [10]. Given that multiple pathologies are associated with AD, the development of novel therapeutics that target these multiple pathologies is desirable in AD treatment. Therefore, the search for safer and effective drugs for the treatment of AD is extremely urgent.
Alzheimer’s disease (AD), the most common form of dementia in the elderly, is characterized by the progressive deterioration of cognitive function and memory [1]. Approximately 50 million people were affected by AD worldwide in 2019, and nearly 10 million new cases occur every year [2]. The Chinese Center for Disease Control and Prevention estimated that there are more than 9 million AD patients in China at present, and it is expected that 40 million could be affected by 2050. AD not only brings a heavy mental burden and economic pressure to the family members and friends of patients but also places a very large burden on the social economy [3]. At present, the typical events in AD pathogenesis are considered to be the formation of extracellular amyloid-β (Aβ) plaques, intracellular accumulation of abnormally phosphorylated tau, neuronal synaptic dysfunction, and neuronal loss [4][5][6][7]. However, over the last decade, all clinical trials targeting a single target, such as Aβ or tau, have failed, and there are also no effective drugs for the prevention or treatment of AD in the clinic [8]. The anti-AD drugs currently approved by the Food and Drug Administration are cholinesterase inhibitors: donepezil, rivastigmine, tacrine, galantamine, and the N-methyl D-aspartate (NMDA) receptor antagonist memantine [9]. These drugs are symptomatic treatments and cannot halt the disease course. Moreover, the above drugs also cause a variety of unfavorable effects, including hypertensive crisis, nausea, diarrhea, and vomiting [10]. Given that multiple pathologies are associated with AD, the development of novel therapeutics that target these multiple pathologies is desirable in AD treatment. Therefore, the search for safer and effective drugs for the treatment of AD is extremely urgent.
Chinese herbal medicine has been widely used for dementia [11].
Uncaria rhynchophylla
(UR), known as “
Gou-Teng” in Chinese, has been used in traditional medicine in Asia, Africa and South America. Traditional Chinese medicine products or formulae containing UR have been used mainly to treat cardiovascular and central nervous system diseases, such as neurodegenerative diseases, lightheadedness, dizziness, convulsions, numbness, and hypertension [12,13,14,15]. Clinically, phytochemicals containing UR, such as Yigan-san (Yokukansan in Japanese), Tianmagouteng granules, and Gouteng-san, have become better herbal medicines for the treatment of stroke, hypertension, and chronic headache in China and Japan. The effects of UR are largely attributed to its predominant active component alkaloids. Therefore, the protective effects of UR and its main alkaloids on the central nervous system diseases have become a focus of research in recent decades.
” in Chinese, has been used in traditional medicine in Asia, Africa and South America. Traditional Chinese medicine products or formulae containing UR have been used mainly to treat cardiovascular and central nervous system diseases, such as neurodegenerative diseases, lightheadedness, dizziness, convulsions, numbness, and hypertension [12][13][14][15]. Clinically, phytochemicals containing UR, such as Yigan-san (Yokukansan in Japanese), Tianmagouteng granules, and Gouteng-san, have become better herbal medicines for the treatment of stroke, hypertension, and chronic headache in China and Japan. The effects of UR are largely attributed to its predominant active component alkaloids. Therefore, the protective effects of UR and its main alkaloids on the central nervous system diseases have become a focus of research in recent decades.
UR is enriched in alkaloids, including rhynchophylline, isorhynchophylline, corynoxeine, isocorynoxeine, and hirsutine [16,17]. The total alkaloid content in UR is approximately 0.2%, among which rhynchophylline accounts for 28–50%, and isorhynchophylline accounts for 15% [18]. In 5xFAD mice, a transgenic mouse model of AD, UR ethanol extract (including rhynchophylline) significantly reduced Aβ aggregation and ameliorated AD-related pathologies (neuronal loss, synaptic degeneration, neuroinflammation, and neurogenesis) [19]. Rhynchophylline (bilateral hippocampal injection, 100 μM, 2 μL) was shown to improve soluble Aβ
UR is enriched in alkaloids, including rhynchophylline, isorhynchophylline, corynoxeine, isocorynoxeine, and hirsutine [16][17]. The total alkaloid content in UR is approximately 0.2%, among which rhynchophylline accounts for 28–50%, and isorhynchophylline accounts for 15% [18]. In 5xFAD mice, a transgenic mouse model of AD, UR ethanol extract (including rhynchophylline) significantly reduced Aβ aggregation and ameliorated AD-related pathologies (neuronal loss, synaptic degeneration, neuroinflammation, and neurogenesis) [19]. Rhynchophylline (bilateral hippocampal injection, 100 μM, 2 μL) was shown to improve soluble Aβ
1-42
-induced impairment of spatial cognition function by inhibiting excessive activation of extrasynaptic NR2B-containing NMDA receptors [20]. Treatment with isorhynchophylline (20 or 40 mg/kg/day) by gavage for 21 days improved Aβ
25-35
-induced cognitive impairment in rats
via
inhibition of neuronal apoptosis and tau protein hyperphosphorylation [21]. A recent study suggested that isorhynchophylline (20 or 40 mg/kg/day) treatment for 4 months improved cognitive impairment in TgCRND8 transgenic mice by reducing Aβ generation and deposition, tau hyperphosphorylation and neuroinflammation [22]. Moreover, 8 weeks of oral treatment with isorhynchophylline (20 or 40 mg/kg/day) could ameliorate D-galactose-induced learning and memory impairments by enhancing the antioxidant status and anti-inflammatory effect of D-galactose in brain tissues via NF-κB signaling [23]. Most of the mentioned studies focus on the therapeutic effects of UR alkaloids, only a few reports have shown that UR alkaloids can affect MAPT, GSK3B, AKT, BCL2, ect. Despite a series of studies on the biological activities of alkaloids in UR, the potential targets and underlying molecular mechanism of UR alkaloids in AD remain largely unclear. Due to the good curative effects of UR alkaloids on AD in animal researches and a variety of pathological processes involved, we therefore hypothesized that UR alkaloids possibly exert an AD treatment effect by acting on multiple pathological processes in AD.
2. The Chemical Structure and ADME Properties of the Main Alkaloids from UR
This study primarily focused on the therapeutic effect of UR alkaloids against AD. A recent study identified 10 alkaloids from UR based on HPLC [17]. The extraction rate of the UR alkaloids (100 g) was 9.4%. The chemical structures of the main alkaloids were obtained from the PubChem database and are shown in
. Next, we used an online tool SwissADME for an in-depth evaluation of the ADME-related properties of the main alkaloids from UR. SwissADME prediction showed that all the main alkaloids satisfied Lipinski’s rule of five, and other chemical and pharmacological properties, including topological polar surface area (TPSA) and solubility (LogS), were also evaluated. These results indicate that these alkaloids may exhibit good permeability across cell membranes.
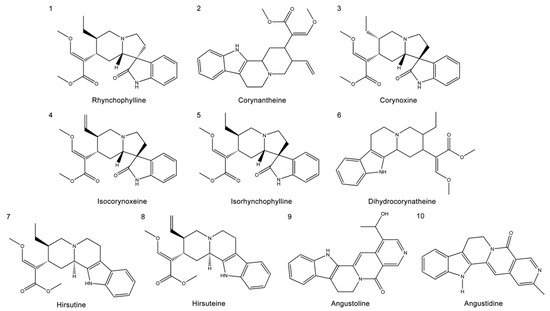
Structures of the main alkaloids extracted from
(UR).
3. Screening of Targets of the Main Alkaloids from UR in AD
Potential targets of the 10 alkaloids were predicted via the SwissTargetPrediction database based on their structure, and a total of 365 targets were obtained. Results from the GeneCards, DrugBank, Therapeutic Target Database (TTD), and Chemogenomics Database for Alzheimer’s Disease identified a total of 622 targets relevant to AD. Ultimately, a Venn diagram was used to summarize 90 common targets associated with both UR alkaloids and AD for further analysis (
A). Furthermore, these 90 target proteins of UR alkaloids against AD were categorized into 6 different classes based on their cellular functions, of which protein-modifying enzyme (PC00260, 34.4%) was the most enriched class (
B). Among these protein-modifying enzymes, AKT1, CDK1, CDK5, EIF2AK3, GSK3B, MAPK10, MTOR and DYRK1A are nonreceptor serine/threonine protein kinases, and ECE1, MME, MMP2, MMP3 and MMP9 are metalloproteases (
C). The above results indicated that alkaloids from UR can protect against AD through multiple targets and biological functions.
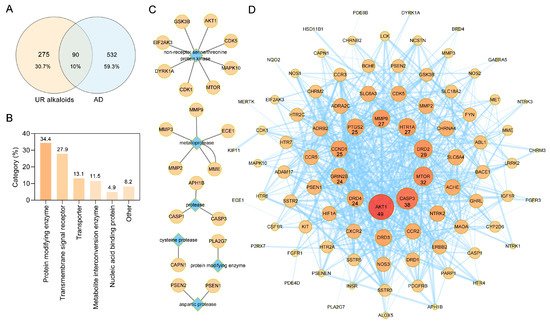
Protein–protein interaction (PPI) network construction for target proteins of UR alkaloids against Alzheimer’s disease (AD). (
) A Venn diagram was applied to obtain the intersection between the 10 UR alkaloids and AD targets. (
) Panther classification was used categorize common targets of UR alkaloids against AD. (
) Target proteins involved in protein modification as enzyme (PC00260). (
) PPI network of UR alkaloids against AD. Nodes represent target proteins, and edges represent interactions among targets. The numbers below the nodes indicate the degree. The darker the color and the larger the node are, the greater the degree is.
4. PPI Analysis of Targets of the Main Alkaloids against AD
To explore the relationship between these 90 targets of alkaloids against AD, protein–protein interaction (PPI) analysis was performed using the STRING database [25]. A PPI network with a total of 90 nodes and 610 edges and an average node degree of 13.6 was generated (
To explore the relationship between these 90 targets of alkaloids against AD, protein–protein interaction (PPI) analysis was performed using the STRING database [24]. A PPI network with a total of 90 nodes and 610 edges and an average node degree of 13.6 was generated (
D). The darker the color and the larger the node were, the greater the degree was. AKT1, CASP3, MTOR, DRD2, HTR1A, MMP9, PTGS2, CCND1, GRIN2B, and DRD4, which are ranked by degree, were identified as core targets (
D). Among these, AKT1 showed the highest degree (49). This demonstrated that these core targets are closely related to other targets in the PPI network, suggesting that these targets may play important roles in AD treatment.
5. Discussion
As the predominant active pharmacological components in UR, UR alkaloids have been widely used as an intervention for neurodegenerative diseases in animal models for several years [12,15,17,19,20,21]. UR alkaloids are mainly administered by oral administration of monomers in the experiments above mentioned. Unfortunately, the potential targets UR alkaloids are not clear at present. This study comprehensively investigated the therapeutic effect of UR alkaloids on AD.
As the predominant active pharmacological components in UR, UR alkaloids have been widely used as an intervention for neurodegenerative diseases in animal models for several years [12][15][17][19][20][21]. UR alkaloids are mainly administered by oral administration of monomers in the experiments above mentioned. Unfortunately, the potential targets UR alkaloids are not clear at present. This study comprehensively investigated the therapeutic effect of UR alkaloids on AD.
Generally, network pharmacology studies use public databases to obtain the main components in traditional Chinese Medicine. Conventional screening methods of public databases do not take into account the content and distribution specificity of components, which will lead to a large number of non-specific components to be screened out. For instance, beta-sitosterol exists widely in 567 kinds of herbs and stigmasterol 284 kinds of herbs (data from HERB,
(accessed on 30 March 2021)). UR alkaloids have significant pharmacological activities and are also representative active ingredients in UR. Accordingly, alkaloids in UR were obtained by HPLC based on a recent study [17], this greatly improved the quality of the data collected. In the present study, all of the 10 alkaloids in UR were in agreement with Lipinski’s rule of five, demonstrating that the alkaloids have acceptable pharmacokinetic properties. Furthermore, 90 potential targets of these 10 alkaloids against AD were obtained using network pharmacology strategies. A potential alkaloid target-AD target network indicated that corynoxine, corynantheine, isorhynchophylline, dihydrocorynatheine, isocorynoxeine, and hirsuteine are likely to become key components for AD treatment. Isorhynchophylline, isocorynoxeine, and hirsuteine showed high permeability, with apparent permeability coefficient values for bidirectional transport at the 10
−5 cm/s level [27]. Corynoxine, a natural neuroprotective autophagy enhancer [28], has the greatest number of therapeutic targets for AD (degree = 48). Corynoxine can regulate several kinases, including RPS6KB1, MAP2K2, and PLK1, contributing to the clearance of AD-associated β-amyloid precursor protein (APP) and Parkinson disease-associated α-synuclein [29]. The molecular docking results also showed that corynoxine strongly binds CCR5, MMP3, CCND1, and NTRK2. Treatment with isorhynchophylline (20 or 40 mg/kg/day) could ameliorate Aβ
cm/s level [25]. Corynoxine, a natural neuroprotective autophagy enhancer [26], has the greatest number of therapeutic targets for AD (degree = 48). Corynoxine can regulate several kinases, including RPS6KB1, MAP2K2, and PLK1, contributing to the clearance of AD-associated β-amyloid precursor protein (APP) and Parkinson disease-associated α-synuclein [27]. The molecular docking results also showed that corynoxine strongly binds CCR5, MMP3, CCND1, and NTRK2. Treatment with isorhynchophylline (20 or 40 mg/kg/day) could ameliorate Aβ
25-35- or D-galactose-induced cognitive impairment in animal models [21,23]. Furthermore, in vitro experiments demonstrated that isorhynchophylline and rhynchophylline (pretreating 100 μM for 2 h) significantly decreased Aβ-induced cell death, intracellular calcium overload, and tau protein hyperphosphorylation in PC12 cells [30]. Notably, there have been few studies on the treatment of AD with corynantheine and dihydrocorynatheine until now. For future research, more attention might be paid to these alkaloids in the treatment of AD.
- or D-galactose-induced cognitive impairment in animal models [21][23]. Furthermore, in vitro experiments demonstrated that isorhynchophylline and rhynchophylline (pretreating 100 μM for 2 h) significantly decreased Aβ-induced cell death, intracellular calcium overload, and tau protein hyperphosphorylation in PC12 cells [28]. Notably, there have been few studies on the treatment of AD with corynantheine and dihydrocorynatheine until now. For future research, more attention might be paid to these alkaloids in the treatment of AD.
Senile plaques (SPs), neurofibrillary tangles (NFTs), neuronal synaptic dysfunction and neuronal loss are characteristic pathologic changes in AD [7,31]. KEGG pathway enrichment analysis revealed that the Alzheimer disease pathway (hsa05010) was the most significantly enriched pathway in UR alkaloids against AD. Among the associated targets, ADAM17 (an α-secretase), BACE1 (a β-secretase), APH1B, NCSTN, PSEN1, and PSENEN (a γ-secretase) are involved in APP cleavage. In AD brains, ADAM17-positive neurons often colocalize with amyloid plaques and are considered potential therapeutic targets for AD [32]. Moreover, ADAM17 is also involved in the cleavage of many other membrane-bound proteins, especially some inflammatory factors related to microglial activation [33]. BACE1 is a rate-limiting enzyme for Aβ production. The inhibition of BACE1 activity could block one of the earliest pathologic events in AD. Several BACE1 inhibitor drug candidates have advanced to phase 3 clinical trials [34]. γ-Secretase activity is mediated by a multiprotein complex consisting of Presenilin, APH1, PEN2 and Nicastrin (NCT) [35]. PSEN1 mutations are responsible for the majority of familial AD cases, and more than 300 mutations in PSEN1 have been reported [36]. Thus, UR alkaloids could decrease Aβ generation and deposition in the brain through several important drug targets. In addition, targets for regulating tau phosphorylation, such as CAPN1, CDK5 and GSK3B, are involved in the anti-AD effects of the UR alkaloids. CDK5 and GSK3B belong to the nonreceptor serine/threonine protein kinase family. GSK3B is a major tau kinase involved in the development of AD tau pathology [37]. A previous study showed that Aβ accumulation in the AD brain can activate kinases that promote tau phosphorylation, including GSK3B [38]. Interestingly, apart from the targets related to the Alzheimer’s disease pathway, up to 28 out of 90 targets were significantly correlated with tau, Aβ, or Aβ and tau. Among these targets, the response to amyloid-beta (GO:1904645) was the most significantly enriched, and the synaptic signaling term (GO:0099536) exhibited the highest number of target connections. CCR5, a cytokine belonging to the β chemokine receptor family of integral membrane proteins, is significantly positively associated with both Aβ and tau pathology. CCR5 expression is strongly related to microglia and inflammation, which accelerate the development of AD [39]. Of the 28 targets related to Aβ and tau pathology, CCR5 had the highest degree in the PPI network and was significantly increased in the hippocampus of AD patients. We should pay more attention to CCR5 in future studies. In addition to affecting Aβ and tau pathology, a series of synaptic-related KEGG pathways, such as serotonergic synapses (hsa04726), dopaminergic synapses (hsa04728), and cholinergic synapses (hsa04725), were also enriched. Cholinergic synapses are ubiquitous in the human central nervous system. The cholinergic hypothesis of AD centers on the progressive loss of limbic and neocortical cholinergic innervation [40]. Cholinesterase inhibitors increase the availability of acetylcholine at synapses in the brain and are one of the few drug therapies that have been proven clinically useful in the treatment of AD, thus validating the cholinergic system as an important therapeutic target in this disease. Overall, our findings indicate that UR alkaloids can directly treat AD by acting on multiple pathological processes in AD.
Senile plaques (SPs), neurofibrillary tangles (NFTs), neuronal synaptic dysfunction and neuronal loss are characteristic pathologic changes in AD [7][29]. KEGG pathway enrichment analysis revealed that the Alzheimer disease pathway (hsa05010) was the most significantly enriched pathway in UR alkaloids against AD. Among the associated targets, ADAM17 (an α-secretase), BACE1 (a β-secretase), APH1B, NCSTN, PSEN1, and PSENEN (a γ-secretase) are involved in APP cleavage. In AD brains, ADAM17-positive neurons often colocalize with amyloid plaques and are considered potential therapeutic targets for AD [30]. Moreover, ADAM17 is also involved in the cleavage of many other membrane-bound proteins, especially some inflammatory factors related to microglial activation [31]. BACE1 is a rate-limiting enzyme for Aβ production. The inhibition of BACE1 activity could block one of the earliest pathologic events in AD. Several BACE1 inhibitor drug candidates have advanced to phase 3 clinical trials [32]. γ-Secretase activity is mediated by a multiprotein complex consisting of Presenilin, APH1, PEN2 and Nicastrin (NCT) [33]. PSEN1 mutations are responsible for the majority of familial AD cases, and more than 300 mutations in PSEN1 have been reported [34]. Thus, UR alkaloids could decrease Aβ generation and deposition in the brain through several important drug targets. In addition, targets for regulating tau phosphorylation, such as CAPN1, CDK5 and GSK3B, are involved in the anti-AD effects of the UR alkaloids. CDK5 and GSK3B belong to the nonreceptor serine/threonine protein kinase family. GSK3B is a major tau kinase involved in the development of AD tau pathology [35]. A previous study showed that Aβ accumulation in the AD brain can activate kinases that promote tau phosphorylation, including GSK3B [36]. Interestingly, apart from the targets related to the Alzheimer’s disease pathway, up to 28 out of 90 targets were significantly correlated with tau, Aβ, or Aβ and tau. Among these targets, the response to amyloid-beta (GO:1904645) was the most significantly enriched, and the synaptic signaling term (GO:0099536) exhibited the highest number of target connections. CCR5, a cytokine belonging to the β chemokine receptor family of integral membrane proteins, is significantly positively associated with both Aβ and tau pathology. CCR5 expression is strongly related to microglia and inflammation, which accelerate the development of AD [37]. Of the 28 targets related to Aβ and tau pathology, CCR5 had the highest degree in the PPI network and was significantly increased in the hippocampus of AD patients. We should pay more attention to CCR5 in future studies. In addition to affecting Aβ and tau pathology, a series of synaptic-related KEGG pathways, such as serotonergic synapses (hsa04726), dopaminergic synapses (hsa04728), and cholinergic synapses (hsa04725), were also enriched. Cholinergic synapses are ubiquitous in the human central nervous system. The cholinergic hypothesis of AD centers on the progressive loss of limbic and neocortical cholinergic innervation [38]. Cholinesterase inhibitors increase the availability of acetylcholine at synapses in the brain and are one of the few drug therapies that have been proven clinically useful in the treatment of AD, thus validating the cholinergic system as an important therapeutic target in this disease. Overall, our findings indicate that UR alkaloids can directly treat AD by acting on multiple pathological processes in AD.
Akt is a serine/threonine protein kinase and an important kinase downstream of PI3K. There are three AKT isoforms (AKT1-3), among which AKT1 is the most important subtype. In this study, the PI3K-Akt signaling pathway (hsa04151) was found by KEGG pathway enrichment analysis to be an important pathway by which UR alkaloids counteract AD, and 17 targets are involved (including the core targets AKT1, CCND1, and MTOR). Among signal transduction pathways, this pathway is a key mediator that regulates cell growth, inflammation, metabolism, and cell survival in response to growth factors. Previous studies have found that Aβ oligomers inhibit the PI3K-Akt pathway, which leads to neuronal death [41]. In the AD brain, normalized pT308 AKT1 was positively correlated with both the amyloid burden and tau tangle density [42]. The PI3K-Akt signaling pathway plays an important role in GSK3B activity regulation, as AKT promotes the phosphorylation of GSK3B, resulting in GSK3B inactivation [43,44]. Furthermore, the core target CASPS (degree = 38) was also involved in AD treatment. CASP3 is a pivotal executioner of apoptosis that plays a major role in neuronal death during nervous system development and under certain pathological conditions. Neuronal cell injury and loss are major neuropathological features of AD. The level of activated CASP3 was elevated in the brains of patients with severe definitive AD [45]. The positive regulation of cell death (GO:0010942) and neuron death (GO:0070997) were also significantly enriched in the present study.
Akt is a serine/threonine protein kinase and an important kinase downstream of PI3K. There are three AKT isoforms (AKT1-3), among which AKT1 is the most important subtype. In this study, the PI3K-Akt signaling pathway (hsa04151) was found by KEGG pathway enrichment analysis to be an important pathway by which UR alkaloids counteract AD, and 17 targets are involved (including the core targets AKT1, CCND1, and MTOR). Among signal transduction pathways, this pathway is a key mediator that regulates cell growth, inflammation, metabolism, and cell survival in response to growth factors. Previous studies have found that Aβ oligomers inhibit the PI3K-Akt pathway, which leads to neuronal death [39]. In the AD brain, normalized pT308 AKT1 was positively correlated with both the amyloid burden and tau tangle density [40]. The PI3K-Akt signaling pathway plays an important role in GSK3B activity regulation, as AKT promotes the phosphorylation of GSK3B, resulting in GSK3B inactivation [41][42]. Furthermore, the core target CASPS (degree = 38) was also involved in AD treatment. CASP3 is a pivotal executioner of apoptosis that plays a major role in neuronal death during nervous system development and under certain pathological conditions. Neuronal cell injury and loss are major neuropathological features of AD. The level of activated CASP3 was elevated in the brains of patients with severe definitive AD [43]. The positive regulation of cell death (GO:0010942) and neuron death (GO:0070997) were also significantly enriched in the present study.
A total of 10 alkaloids in UR and 90 common targets against AD were screened by network pharmacology analysis. GO enrichment and KEGG pathway enrichment analyses suggested that UR alkaloids can directly treat AD by acting on multiple AD pathological processes, such as processes involving Aβ and tau, neuronal synaptic function, and neuronal loss. However, clinical studies are necessary to verify these theoretical observations in the future. The bioavailability of UR alkaloids after oral administration was not reported in the clinic. The oral bioavailability of rhynchophylline, isorhynchophylline, hirsutine, and angustidine was 41.82%, 12.71%, 34.44%, and 51.85%, respectively, based on the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP,
(accessed on 30 March 2021)). It is necessary to further optimize the structure or improve the dosage form to increase the oral bioavailability for its clinical applicability. Taken together, our findings might provide a theoretical basis for the use of UR alkaloids as a therapeutic for AD.
References
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944.
- Zhang, F.; Zhong, R.J.; Cheng, C.; Li, S.; Le, W.D. New therapeutics beyond amyloid-beta and tau for the treatment of Alzheimer’s disease. Acta Pharmacol. Sin. 2020, 1–8.
- Guo, Y.; Yang, H.; Huang, Z.; Tian, S.; Li, Q.; Du, C.; Chen, T.; Liu, Y.; Sun, H.; Liu, Z. Design, Synthesis, and Evaluation of Acetylcholinesterase and Butyrylcholinesterase Dual-Target Inhibitors against Alzheimer’s Diseases. Molecules 2020, 25, 489.
- Wallace, R.A.; Dalton, A.J. What can we learn from study of Alzheimer’s disease in patients with Down syndrome for early-onset Alzheimer’s disease in the general population? Alzheimers Res. Ther. 2011, 3, 13.
- Asaad, M.; Lee, J.H. A guide to using functional magnetic resonance imaging to study Alzheimer’s disease in animal models. Dis. Model. Mech. 2018, 11, dmm031724.
- Sheng, J.G.; Zhou, X.Q.; Mrak, R.E.; Griffin, W.S. Progressive neuronal injury associated with amyloid plaque formation in Alzheimer disease. J. Neuropathol. Exp. Neurol. 1998, 57, 714–717.
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517.
- Mehta, D.; Jackson, R.; Paul, G.; Shi, J.; Sabbagh, M. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010–2015. Expert Opin. Investig. Drugs 2017, 26, 735–739.
- Melnikova, I. Therapies for Alzheimer’s disease. Nat. Rev. Drug Discov. 2007, 6, 341–342.
- Inglis, F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int. J. Clin. Pract. Suppl. 2002, 127, 45–63.
- Lee, J.; Jin, C.; Cho, S.Y.; Park, S.U.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Cho, K.H.; Kwon, S. Herbal medicine treatment for Alzheimer disease: A protocol for a systematic review and meta-analysis. Medicine 2020, 99, e21745.
- Zhou, J.; Zhou, S. Antihypertensive and neuroprotective activities of rhynchophylline: The role of rhynchophylline in neurotransmission and ion channel activity. J. Ethnopharmacol. 2010, 132, 15–27.
- Hsieh, C.L.; Chen, M.F.; Li, T.C.; Li, S.C.; Tang, N.Y.; Hsieh, C.T.; Pon, C.Z.; Lin, J.G. Anticonvulsant effect of Uncaria rhynchophylla (Miq) Jack. in rats with kainic acid-induced epileptic seizure. Am. J. Chin. Med. 1999, 27, 257–264.
- Hsieh, C.L.; Tang, N.Y.; Chiang, S.Y.; Hsieh, C.T.; Lin, J.G. Anticonvulsive and free radical scavenging actions of two herbs, Uncaria rhynchophylla (MIQ) Jack and Gastrodia elata Bl., in kainic acid-treated rats. Life Sci. 1999, 65, 2071–2082.
- Liu, L.; Zhao, Y.H.; Zeng, C.Q.; Zeng, Y. Research progress in pharmacological effects of Uncaria Hook on Alzheimer disease models. Acta Pharm. Sin. 2016, 51, 536–542.
- Yuan, D.; Ma, B.; Yang, J.Y.; Xie, Y.Y.; Wang, L.; Zhang, L.J.; Kano, Y.; Wu, C.F. Anti-inflammatory effects of rhynchophylline and isorhynchophylline in mouse N9 microglial cells and the molecular mechanism. Int. Immunopharmacol. 2009, 9, 1549–1554.
- Zheng, M.; Chen, M.; Liu, C.; Fan, Y.; Shi, D. Alkaloids extracted from Uncaria rhynchophylla demonstrate neuroprotective effects in MPTP-induced experimental parkinsonism by regulating the PI3K/Akt/mTOR signaling pathway. J. Ethnopharmacol. 2021, 266, 113451.
- Shi, J.S.; Yu, J.X.; Chen, X.P.; Xu, R.X. Pharmacological actions of Uncaria alkaloids, rhynchophylline and isorhynchophylline. Acta Pharmacol. Sin. 2003, 24, 97–101.
- Shin, S.J.; Jeong, Y.; Jeon, S.G.; Kim, S.; Lee, S.K.; Choi, H.S.; Im, C.S.; Kim, S.H.; Kim, S.H.; Park, J.H.; et al. Uncaria rhynchophylla ameliorates amyloid beta deposition and amyloid beta-mediated pathology in 5XFAD mice. Neurochem. Int. 2018, 121, 114–124.
- Yang, Y.; Ji, W.G.; Zhu, Z.R.; Wu, Y.L.; Zhang, Z.Y.; Qu, S.C. Rhynchophylline suppresses soluble Abeta1-42-induced impairment of spatial cognition function via inhibiting excessive activation of extrasynaptic NR2B-containing NMDA receptors. Neuropharmacology 2018, 135, 100–112.
- Xian, Y.F.; Mao, Q.Q.; Wu, J.C.; Su, Z.R.; Chen, J.N.; Lai, X.P.; Ip, S.P.; Lin, Z.X. Isorhynchophylline treatment improves the amyloid-beta-induced cognitive impairment in rats via inhibition of neuronal apoptosis and tau protein hyperphosphorylation. J. Alzheimers Dis. 2014, 39, 331–346.
- Li, H.Q.; Ip, S.P.; Yuan, Q.J.; Zheng, G.Q.; Tsim, K.; Dong, T.; Lin, G.; Han, Y.; Liu, Y.; Xian, Y.F.; et al. Isorhynchophylline ameliorates cognitive impairment via modulating amyloid pathology, tau hyperphosphorylation and neuroinflammation: Studies in a transgenic mouse model of Alzheimer’s disease. Brain Behav. Immun. 2019, 82, 264–278.
- Xian, Y.F.; Su, Z.R.; Chen, J.N.; Lai, X.P.; Mao, Q.Q.; Cheng, C.H.; Ip, S.P.; Lin, Z.X. Isorhynchophylline improves learning and memory impairments induced by D-galactose in mice. Neurochem. Int. 2014, 76, 42–49.
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613.
- Zhang, Y.N.; Yang, Y.F.; Xu, W.; Yang, X.W. The Blood-Brain Barrier Permeability of Six Indole Alkaloids from Uncariae Ramulus Cum Uncis in the MDCK-pHaMDR Cell Monolayer Model. Molecules 2017, 22, 1944.
- Chen, L.L.; Song, J.X.; Lu, J.H.; Yuan, Z.W.; Liu, L.F.; Durairajan, S.S.; Li, M. Corynoxine, a natural autophagy enhancer, promotes the clearance of alpha-synuclein via Akt/mTOR pathway. J. Neuroimmune Pharmacol. 2014, 9, 380–387.
- Chen, L.L.; Wang, Y.B.; Song, J.X.; Deng, W.K.; Lu, J.H.; Ma, L.L.; Yang, C.B.; Li, M.; Xue, Y. Phosphoproteome-based kinase activity profiling reveals the critical role of MAP2K2 and PLK1 in neuronal autophagy. Autophagy 2017, 13, 1969–1980.
- Xian, Y.F.; Lin, Z.X.; Mao, Q.Q.; Hu, Z.; Zhao, M.; Che, C.T.; Ip, S.P. Bioassay-Guided Isolation of Neuroprotective Compounds from Uncaria rhynchophylla against Beta-Amyloid-Induced Neurotoxicity. Evid. Based Complement. Alternat. Med. 2012, 2012, 802625.
- Frey, H.J.; Mattila, K.M.; Korolainen, M.A.; Pirttila, T. Problems associated with biological markers of Alzheimer’s disease. Neurochem. Res. 2005, 30, 1501–1510.
- Skovronsky, D.M.; Fath, S.; Lee, V.M.; Milla, M.E. Neuronal localization of the TNFalpha converting enzyme (TACE) in brain tissue and its correlation to amyloid plaques. J. Neurobiol. 2001, 49, 40–46.
- Qian, M.; Shen, X.; Wang, H. The Distinct Role of ADAM17 in APP Proteolysis and Microglial Activation Related to Alzheimer’s Disease. Cell. Mol. Neurobiol. 2016, 36, 471–482.
- Koelsch, G. BACE1 Function and Inhibition: Implications of Intervention in the Amyloid Pathway of Alzheimer’s Disease Pathology. Molecules 2017, 22, 1723.
- De Strooper, B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron 2003, 38, 9–12.
- Ryan, N.S.; Nicholas, J.M.; Weston, P.; Liang, Y.; Lashley, T.; Guerreiro, R.; Adamson, G.; Kenny, J.; Beck, J.; Chavez-Gutierrez, L.; et al. Clinical phenotype and genetic associations in autosomal dominant familial Alzheimer’s disease: A case series. Lancet Neurol. 2016, 15, 1326–1335.
- Ly, P.T.; Wu, Y.; Zou, H.; Wang, R.; Zhou, W.; Kinoshita, A.; Zhang, M.; Yang, Y.; Cai, F.; Woodgett, J.; et al. Inhibition of GSK3beta-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J. Clin. Investig. 2013, 123, 224–235.
- Ochalek, A.; Mihalik, B.; Avci, H.X.; Chandrasekaran, A.; Teglasi, A.; Bock, I.; Giudice, M.L.; Tancos, Z.; Molnar, K.; Laszlo, L.; et al. Neurons derived from sporadic Alzheimer’s disease iPSCs reveal elevated TAU hyperphosphorylation, increased amyloid levels, and GSK3B activation. Alzheimers Res. Ther. 2017, 9, 90.
- Li, T.; Zhu, J. Entanglement of CCR5 and Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 209.
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933.
- Gabbouj, S.; Ryhanen, S.; Marttinen, M.; Wittrahm, R.; Takalo, M.; Kemppainen, S.; Martiskainen, H.; Tanila, H.; Haapasalo, A.; Hiltunen, M.; et al. Altered Insulin Signaling in Alzheimer’s Disease Brain–Special Emphasis on PI3K-Akt Pathway. Front. Neurosci. 2019, 13, 629.
- Arvanitakis, Z.; Wang, H.Y.; Capuano, A.W.; Khan, A.; Taib, B.; Anokye-Danso, F.; Schneider, J.A.; Bennett, D.A.; Ahima, R.S.; Arnold, S.E. Brain Insulin Signaling, Alzheimer Disease Pathology, and Cognitive Function. Ann. Neurol. 2020, 88, 513–525.
- Grimes, C.A.; Jope, R.S. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog. Neurobiol. 2001, 65, 391–426.
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785–789.
- Stadelmann, C.; Deckwerth, T.L.; Srinivasan, A.; Bancher, C.; Bruck, W.; Jellinger, K.; Lassmann, H. Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer’s disease. Evidence for apoptotic cell death. Am. J. Pathol. 1999, 155, 1459–1466.
