There is increasing evidence of sex differences in the action of anti-inflammatory drugs, with women being at significantly higher risk of adverse effects. Nevertheless, clinicians’ awareness of the implications of these sex differences on dosing and adverse event monitoring in routine practice is still in need of improvement.
- sex
- gender
- NSAID
- anti-inflammatory drug
- pharmacokinetics
- pharmacodynamics
- steroid
- SARS-CoV-2
- COVID-19
1. Introduction
Numerous investigations have documented that women, in general, are dispensed more prescription and over-the-counter (OTC) drugs compared to men [1]. Gender-specific differences may play an important role in pharmacotherapy, which was long underestimated. Furthermore, incidences of several diseases differ between men and women. For instance, women suffer more often and more severely from osteoporosis [2], asthma [3], migraines [4], depression [5], irritable bowel diseases [6], or autoimmune diseases such as rheumatoid arthritis, lupus erythematosus, or multiple sclerosis [7][8]. In contrast, men are more likely to suffer from various forms of cancer e.g., hepatocellular carcinoma [9] or cardiovascular diseases [10]. In this context, it must be noted that women typically display a later onset of coronary heart disease than men [10]. This may be related to cardioprotective effects of oestrogen [10][11].
Numerous investigations have documented that women, in general, are dispensed more prescription and over-the-counter (OTC) drugs compared to men [1]. Gender-specific differences may play an important role in pharmacotherapy, which was long underestimated. Furthermore, incidences of several diseases differ between men and women. For instance, women suffer more often and more severely from osteoporosis [2], asthma [3], migraines [4], depression [5], irritable bowel diseases [6], or autoimmune diseases such as rheumatoid arthritis, lupus erythematosus, or multiple sclerosis [7,8]. In contrast, men are more likely to suffer from various forms of cancer e.g., hepatocellular carcinoma [9] or cardiovascular diseases [10]. In this context, it must be noted that women typically display a later onset of coronary heart disease than men [10]. This may be related to cardioprotective effects of oestrogen [10,11].
In the past, research has focused on the male organism, and women tended to be under-represented in clinical trials, as were female rodent models from pre-clinical research [12][13][14][15]. However, an increasing number of studies have shown sex-specific differences in the effects of drugs which, while mostly pharmacokinetic in nature, can also be caused by pharmacodynamic differences [14][16]. There are also trivial aspects that play a partly decisive role: on average, women have lower body height and weight than men so that a given drug dose leads to a higher concentration of the active ingredient in women. Women have a higher body-fat ratio while their body-water ratio is lower, which has major effects on the concentration, distribution, and effect duration of drugs [16][17]. Hence, lipophilic agents have a higher volume of distribution (Vd) in women, while hydrophilic active ingredients have a lower Vd. Therefore, identical doses of a lipophilic drug led to lower plasma concentrations in women than men, while a hydrophilic one leads to higher plasma concentrations [16].
In the past, research has focused on the male organism, and women tended to be under-represented in clinical trials, as were female rodent models from pre-clinical research [12,13,14,15]. However, an increasing number of studies have shown sex-specific differences in the effects of drugs which, while mostly pharmacokinetic in nature, can also be caused by pharmacodynamic differences [14,16]. There are also trivial aspects that play a partly decisive role: on average, women have lower body height and weight than men so that a given drug dose leads to a higher concentration of the active ingredient in women. Women have a higher body-fat ratio while their body-water ratio is lower, which has major effects on the concentration, distribution, and effect duration of drugs [16,17]. Hence, lipophilic agents have a higher volume of distribution (Vd) in women, while hydrophilic active ingredients have a lower Vd. Therefore, identical doses of a lipophilic drug led to lower plasma concentrations in women than men, while a hydrophilic one leads to higher plasma concentrations [16].
There are also remarkable differences between the sexes regarding the activity of drug metabolizing enzymes, both in phase I (drug functionalization) and phase II (drug conjugation). Additionally, some efflux transporters like P-glycoprotein (P-gp, ABCB1) and the Breast Cancer Resistance Protein (BCRP) are more active in men than in women [18][19][20][21]. Potential mechanisms behind the differential functioning of male and female bodies have been reviewed extensively elsewhere [15][21][22][23].
There are also remarkable differences between the sexes regarding the activity of drug metabolizing enzymes, both in phase I (drug functionalization) and phase II (drug conjugation). Additionally, some efflux transporters like P-glycoprotein (P-gp, ABCB1) and the Breast Cancer Resistance Protein (BCRP) are more active in men than in women [18,19,20,21]. Potential mechanisms behind the differential functioning of male and female bodies have been reviewed extensively elsewhere [15,21,22,23].
Irrespective of the known sex disparities, a uniform pharmacological treatment approach has been followed for men and women, most notably, women are treated with drug doses derived from studies carried out on men. Hence, women suffer twice as often from drug-induced side effects, e.g., drug-induced long QT syndrome [24].
2. Sex-Specific Differences in Immune Responses and Inflammatory Diseases
The body’s immune response, i.e., the body’s protection and defence system against both its own and foreign antigens, is substantially different between the sexes. In general, the body at first responds to tissue damage or pathogen intrusion (e.g., virus, bacteria, fungal infection or parasites) with an acute inflammation. These inflammatory processes serve to mitigate the harmful influence, and to initiate healing and regeneration processes. However, if this fine-tuned cascade spirals out of control, the inflammation cannot be stopped and leads to chronic inflammatory disease [25].
As reviewed extensively by Klein et al., numerous studies have shown remarkable sex disparities regarding the activities of the innate and adaptive immune systems (
) [26].
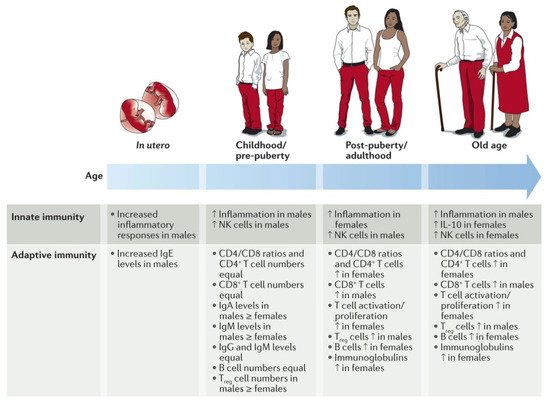
Figure 1.
+
reg
It could be shown, for instance, that elderly women have a more active innate and adaptive immune system than men of the same age [27]. However, age seems to be a key factor as the immune responses and inflammation activities are more pronounced in boys up to puberty but afterwards, are more pronounced in women [28].
Research of the underlying mechanisms has shown very complex differences in numerous immune cells driving the innate and the adaptive immune responses. For innate immunity, it could be shown that the phagocytosis by neutrophils and macrophages, the macrophages’ activity, the type-1 interferon (IFN) activity of the dendritic cells, and the efficiency of the antigen-presenting cells are all more pronounced in female cells than in their male counterparts (
Table 1.
| Immune Component | Characteristic | Sex Difference |
|---|---|---|
| Sex differences in the innate immune system | ||
| TLR pathways | TLR pathway gene expression | Higher in females |
| TLR7 expression | Higher in females | |
| IL-10 production by TLR9-stimulated PBMCs | Higher in males | |
| APCs | APC efficiency | Higher in females |
| Dendritic cells | TLR7 activity | Higher in females |
| Type 1 interferon activity | Higher in females | |
| Macrophages | TLR4 expression | Higher in males |
| Activation | Higher in females | |
| Phagocytic capacity | Higher in females | |
| Pro-inflammatory cytokine production | Higher in males | |
| IL-10 production | Higher in females | |
| Neutrophils | Phagocytic capacity | Higher in females |
| TLR expression | Higher in males | |
| NK cells | NK cell numbers | Higher in males |
| Sex differences in the adaptive immune system | ||
| Thymus | Size of thymus | Larger in males |
| T cells | CD4+ T cell counts | Higher in females |
| CD4/CD8 T cell ratio | Higher in females | |
| CD8+ T cell counts | Higher in males | |
| Number of activated T cells | Higher in females | |
| T cell proliferation | Greater in females | |
| Cytotoxic T cells | Increased cytotoxic activity in females | |
| TH1 versus TH2 cell bias | TH2 cell bias in females, TH1 cell bias in males | |
| Treg cell numbers | Increased in males | |
| B cells | B cell numbers | Increased in females |
| Immunoglobulins | Antibody production | Higher in females |
APC, antigen-presenting cell; IL, interleukin; NK, natural killer; PBMCs, peripheral blood mononuclear cells; TH, T helper; TLR, Toll-like receptor; Treg, regulatory T. * Based on data from humans and rodents and primary cell cultures. Reproduced with permission from [26].
In contrast, men have higher numbers of natural killer (NK) cells and the expression of toll-like receptors (TLR) on macrophages and neutrophils. With regard to acquired immunity, women have more B-lymphocytes and, therefore, better antibody production, higher amounts of active T-lymphocytes with higher proliferation rates, more CD4+ cells, and a pronounced T-cell cytotoxicity. Men, on the other hand, have higher concentrations of regulated T-lymphocytes and CD8+ cells [26].
Due to the more active immune system in women, it is obvious that their inflammatory response will be stronger than mens. Various pro-inflammatory processes, e.g., the release of the immune-stimulating IFN-γ and interleukin (IL)-17 by T-lymphocytes or the plasma levels of cytokines such as tumour necrosis factor (TNF) and IL-6, are more pronounced in women [26]. On the other hand, the release of the inflammation-inhibiting cytokines IL-4 and IL-10 following the leukocyte stimulation are more pronounced in men.
Due to the more active immune system in women, it is obvious that their inflammatory response will be stronger than mens. Various pro-inflammatory processes, e.g., the release of the immune-stimulating IFN-γ and interleukin (IL)-17 by T-lymphocytes or the plasma levels of cytokines such as tumour necrosis factor (TNF) and IL-6, are more pronounced in women [26]. On the other hand, the release of the inflammation-inhibiting cytokines IL-4 and IL-10 following the leukocyte stimulation are more pronounced in men.
Differences in male and female immune responses were also observed in patients who had acquired SARS-CoV-2 [29]. Male sex is associated with more hospitalizations, more severe course of disease, and higher mortality [30]. Studies found lower T-cell frequencies and activation in men [31,32], downregulation of B-cell activity and NK cell activating receptors in men [32], as well as a predominance of men among patients with neutralizing IgG autoantibodies against IFN-ω and/or IFN-α, enabling easier SARS-CoV-2 infections [33,34,35]. Sex differences in immune responses have been shown to cause differences in vaccine effectiveness [35,36]. Interestingly, analyses for sex differences are mostly lacking or of a rudimentary level in reports of trials investigating antiviral drugs, corticosteroids, and–importantly–vaccines [35,36,37].
Differences in male and female immune responses were also observed in patients who had acquired SARS-CoV-2 [29]. Male sex is associated with more hospitalizations, more severe course of disease, and higher mortality [30]. Studies found lower T-cell frequencies and activation in men [31][32], downregulation of B-cell activity and NK cell activating receptors in men [32], as well as a predominance of men among patients with neutralizing IgG autoantibodies against IFN-ω and/or IFN-α, enabling easier SARS-CoV-2 infections [33][34][35]. Sex differences in immune responses have been shown to cause differences in vaccine effectiveness [35][36]. Interestingly, analyses for sex differences are mostly lacking or of a rudimentary level in reports of trials investigating antiviral drugs, corticosteroids, and–importantly–vaccines [35][36][37].
Sex discrepancies between the innate and acquired immune responses are not only caused by their sex chromosomes but also by differences in sexual hormones [38]. Many genes on the female X-chromosome (e.g., genes for TLR) regulate immune function and contribute to the predominance of autoimmune diseases in women [26,38]. Polymorphisms of the male Y-chromosome contribute to the higher susceptibility to viral infections. The so-called Klinefelter syndrome in men is caused by an extra X-chromosome and is associated with a reduced testosterone level and increased oestrogen levels [39]. Men with Klinefelter syndrome develop autoimmune diseases more often, and their immune response is similar to that of women. For example, the immunoglobulin concentration and numbers of B-cells and CD4+-T-cells in men with Klinefelter syndrome are elevated but can be reduced through testosterone therapy [40].
Sex discrepancies between the innate and acquired immune responses are not only caused by their sex chromosomes but also by differences in sexual hormones [38]. Many genes on the female X-chromosome (e.g., genes for TLR) regulate immune function and contribute to the predominance of autoimmune diseases in women [26][38]. Polymorphisms of the male Y-chromosome contribute to the higher susceptibility to viral infections. The so-called Klinefelter syndrome in men is caused by an extra X-chromosome and is associated with a reduced testosterone level and increased oestrogen levels [39]. Men with Klinefelter syndrome develop autoimmune diseases more often, and their immune response is similar to that of women. For example, the immunoglobulin concentration and numbers of B-cells and CD4+-T-cells in men with Klinefelter syndrome are elevated but can be reduced through testosterone therapy [40].
Oestradiol stimulates the inflammation process in accordance with the higher inflammation activity of the female immune cells, while testosterone acts on inflammation inhibitors [38,41]. Half of the genes in activated T-cells have response elements for oestrogen receptors. Testosterone is generally classified as antinociceptive while oestradiol and progesterone can act as a pro- as well as an antinociceptive agents. In experiments using castrated rats, oestradiol substitution reduced the pain threshold while testosterone substitution increased it [42]. Interestingly, high oestradiol levels in patients with rheumatoid arthritis suppressed the pain threshold while lower concentrations stimulated it [43]. Oestradiol increases the formation of immunoglobulin, the release of IFN-γ from leukocytes and the activity of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in T-lymphocytes [26,43]. Testosterone reduces elevated biosynthetic pro-inflammatory leukotriene levels in granulocytes and monocytes, and high testosterone plasma levels correlate with lower leukotriene formation in women [44].
Oestradiol stimulates the inflammation process in accordance with the higher inflammation activity of the female immune cells, while testosterone acts on inflammation inhibitors [38][41]. Half of the genes in activated T-cells have response elements for oestrogen receptors. Testosterone is generally classified as antinociceptive while oestradiol and progesterone can act as a pro- as well as an antinociceptive agents. In experiments using castrated rats, oestradiol substitution reduced the pain threshold while testosterone substitution increased it [42]. Interestingly, high oestradiol levels in patients with rheumatoid arthritis suppressed the pain threshold while lower concentrations stimulated it [43]. Oestradiol increases the formation of immunoglobulin, the release of IFN-γ from leukocytes and the activity of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in T-lymphocytes [26][43]. Testosterone reduces elevated biosynthetic pro-inflammatory leukotriene levels in granulocytes and monocytes, and high testosterone plasma levels correlate with lower leukotriene formation in women [44].
In addition to influencing the pathophysiology of inflammation, sex hormones affect the pharmacodynamics and pharmacokinetics of drugs [21]. For example, oestradiol slows gastric emptying, increases the body-fat ratio, and reduces the amount of α1-glycoprotein, which non-specifically binds alkaline drugs. Furthermore, sex hormones such as oestrogen (primarily as oestradiol) in women and androgens (primarily testosterone) in men are directly or indirectly involved in many transmembrane transport processes [21].
In addition to influencing the pathophysiology of inflammation, sex hormones affect the pharmacodynamics and pharmacokinetics of drugs [21]. For example, oestradiol slows gastric emptying, increases the body-fat ratio, and reduces the amount of α1-glycoprotein, which non-specifically binds alkaline drugs. Furthermore, sex hormones such as oestrogen (primarily as oestradiol) in women and androgens (primarily testosterone) in men are directly or indirectly involved in many transmembrane transport processes [21].
Taken together, these findings have profound consequences on the defence mechanisms against infections, autoimmune diseases, malignant diseases, and on vaccinations.
Taken together, these findings have profound consequences on the defence mechanisms against infections, autoimmune diseases, malignant diseases, and on vaccinations.
