Osteocytes are the most abundant bone cells, entrapped inside the mineralized bone matrix. They derive from osteoblasts through a complex series of morpho-functional modifications; such modifications not only concern the cell shape (from prismatic to dendritic) and location (along the vascular bone surfaces or enclosed inside the lacuno-canalicular cavities, respectively) but also their role in bone processes (secretion/mineralization of preosseous matrix and/or regulation of bone remodeling). Osteocytes are connected with each other by means of different types of junctions, among which the gap junctions enable osteocytes inside the matrix to act in a neuronal-like manner, as a functional syncytium together with the cells placed on the vascular bone surfaces (osteoblasts or bone lining cells), the stromal cells and the endothelial cells, i.e., the bone basic cellular system (BBCS). Within the BBCS, osteocytes can communicate in two ways: by means of volume transmission and wiring transmission, depending on the type of signals (metabolic or mechanical, respectively) received and/or to be forwarded. The capability of osteocytes in maintaining skeletal and mineral homeostasis is due to the fact that it acts as a mechano-sensor, able to transduce mechanical strains into biological signals and to trigger/modulate the bone remodeling, also because of the relevant role of sclerostin secreted by osteocytes, thus regulating different bone cell signaling pathways.
- osteocytes
- bone mechano-sensor
- skeletal homeostasis
- mineral homeostasis
- bone remodeling
1. Introduction
It has been known for more than a century [1][2][3] that the osteocyte originates from the osteoblast. However, the process of osteoblast-to-osteocyte differentiation has been widely investigated only at a later time point with regard to both morphological and functional aspects. The structural differences between osteoblasts and osteocytes were shown by various authors in the 1960s to1980s [4][5][6][7][8], but only afterwards was established the temporal sequence of the events that allows the transformation of the prismatic osteoblast (generally arranged in laminae facing the vascular bone surfaces) into the dendritic mature osteocyte (embedded in the mineralized matrix) [9][10][11]. Concerning the dynamic modification of the cell body of preosteocyte (i.e., the differentiating osteocyte), the amount of the cytoplasmic organelles decreases, whereas the nucleus-to-cytoplasm ratio increases depending on the diminution of its secretive activity [9]. In parallel to both the cellular body reduction in size and the modification in ultrastructure, the formation of the cytoplasmic processes proceeds with an asynchronous and asymmetric pattern, considering that the cells in differentiation are progressively further away from the vascular surface due to the osteoid secreted by the osteoblastic lamina (Figure 1): firstly, the differentiating osteocytes maintain contacts with the mature osteocytes that recruited them from the osteoblastic lamina, forming short “mineral” processes; later, they establish contacts with the migrating osteoblastic lamina, elongating slender and long “vascular” processes, issued before the complete mineralization of the surrounding osteoid. The asynchronous and asymmetrical dendrogenesis is the expression of the fact that osteocytes (as all bone cells) live in an asymmetrical environment, between the mature mineralized matrix and the vascular surface (covered by osteoblastic laminae or bone lining cells); thus, it is logical to expect that not only the osteocytes, but also the preosteocytes and the osteoblasts, are morpho-functionally asymmetric cells.
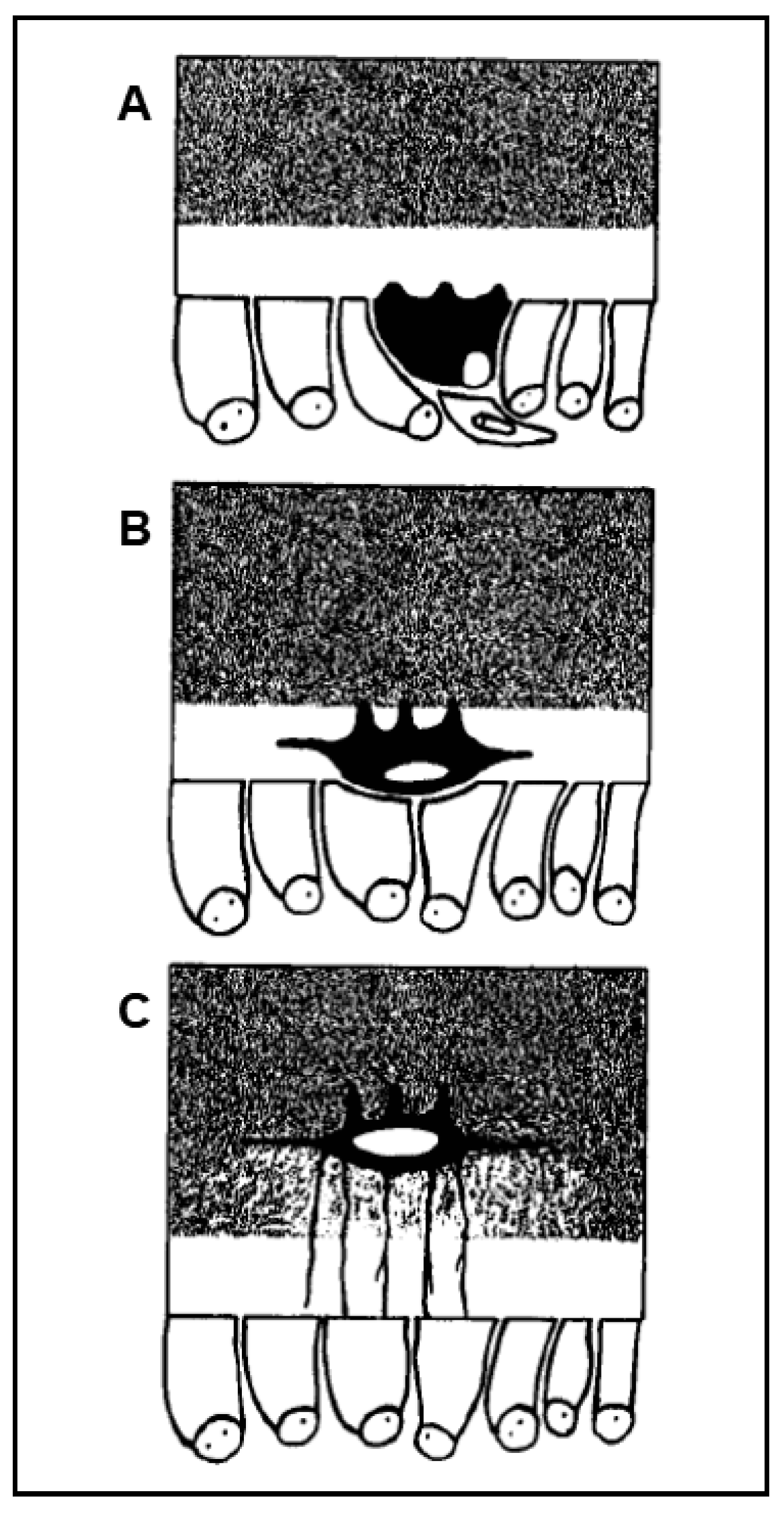
Figure 1.
A
B
C
At the end of the process, the osteocytes are confined to lacuno-canalicular cavities, “prisoners” inside the mineralized matrix. Despite this fact, they are connected, thanks to the dendrogenesis process, to each other and with the bone cells on the vascular surfaces through a network of dendrities, running within the canalicular network; this condition allows osteocytes to act as “orchestrators” of bone processes [12][13]. Prerequisite for that is the existence of junctional complexes occurring among osteocyte cytoplasmic processes [7][14][15], suggesting that the bone cells of the osteogenic lineage, arranged in network (Figure 2), might act as a functional syncytium, that includes also the cells covering the vascular bone surfaces, bone lining cells [15] or osteoblasts [16].
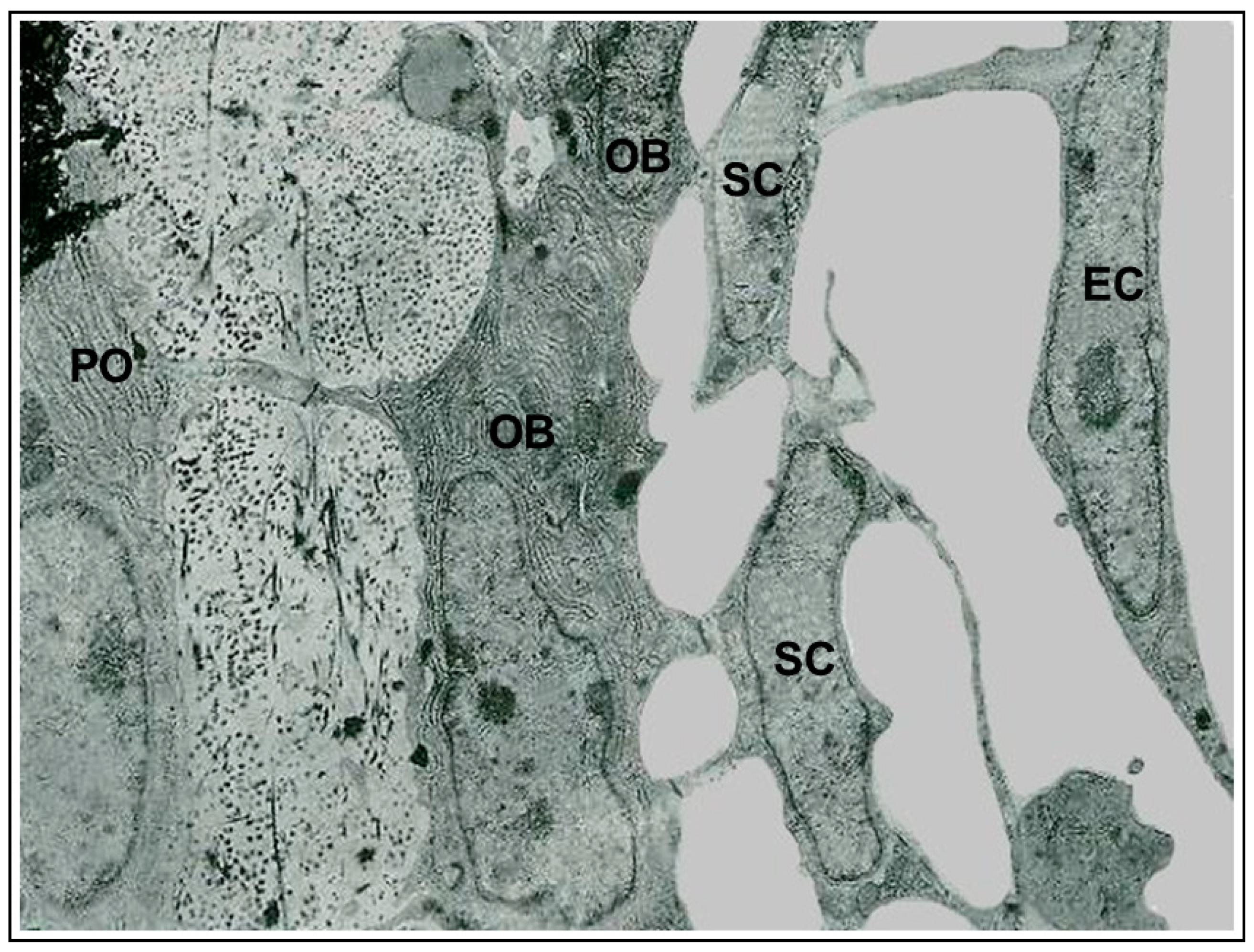
Figure 2.
In conclusion, throughout the whole differentiation process, preosteocytes are always in close relationship with the neighboring cells (osteoblasts, osteocytes) by means of variously-shaped intercellular contacts (invaginated finger-like, side-to-side, and end to-end) and two types of specialized junctions: gap and adherens [14]. The pivotal role played by these contacts and junctions in osteocyte differentiation and activity will be discussed in the context of their distinct functional significance.
2. Interplay between Mineral and Skeletal Homeostasis and Osteocyte Role Mediated by Sclerostin
[22]
[21]
[20]
[33]
3. Conclusions
In conclusion, despite the “segregation” within the mineralized bone matrix, the osteocyte is the dynamic bone cellular element that triggers/guides/modulates a series of sophisticated and interconnected processes, in hierarchic priority: their balance is allowed by osteocyte capability to sense the different bone demands (i.e., metabolic, hormonal, mechanical, etc.) and, depending on the interaction with the actual systemic conditions, to act on the “operator” bone cells that form and destroyed bone tissue under the direction of the best orchestrator.
References
- Gegenbaur, C. Über die Bildung des Knochengewebes I u II. Z. Naturwiss 1864, 1, 343–369.
- Waldeyer, W. Über den Ossifikationsprozess. Arch. Mikrosk. Anat. Entw. Mech. 1865, 1, 354–375.
- Waldeyer, W. Über den Ossifikationsprozess. Zentbl. Med. Wiss 1865, 6, 113–116.
- Dudley, H.R.; Spiro, D. The fine structure of bone cells. J. Biophys. Biochem. Cytol. 1961, 11, 627–649.
- Hancox, N.M.; Boothroyd, B. Electron microscopy of the early stages of osteogenesis. Clin. Orthop. 1965, 40, 153–161.
- Cameron, D.A. The ultrastructure of bone. In The Biochemistry and Physiology of Bone; Bourne, G.H., Ed.; Academic Press: New York, NY, USA, 1972; Volume I, pp. 191–236.
- Rasmussen, H.; Bordier, P. The Physiological and Cellular Basis of Metabolic Bone Disease; The William & Wilkins Company: Baltimore, MD, USA, 1974.
- Njweide, P.J.; van der Plas, A.; Scherft, J.P. Biochemical and histological studies on various bone cell preparation. Calcif. Tissue Int. 1981, 33, 529–540.
- Palumbo, C. A three-dimensional ultrastructural study of osteoid-osteocytes in the tibia of chick embryos. Cell Tissue Res. 1986, 246, 125–131.
- Marotti, G.; Cane, V.; Palazzini, S.; Palumbo, C. Structure-function relationships in the osteocyte. Ital. J. Miner. Electrolyte Metab. 1990, 4, 93–106.
- Palumbo, C.; Palazzini, S.; Zaffe, D.; Marotti, G. Osteocyte differentiation in the tibia of newborn rabbit: An ultrastructural study of the formation of cytoplasmic processes. Acta Anat. 1990, 137, 350–358.
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238.
- Schaffler, M.B.; Cheung, W.Y.; Majeska, R.; Kennedy, O. Osteocytes: Master orchestrators of bone. Calcif. Tissue Int. 2014, 94, 5–24.
- Palumbo, C.; Palazzini, S.; Marotti, G. Morphological study of intercellular junctions during osteocyte differentiation. Bone 1990, 11, 401–406.
- Miller, S.C.; Bowman, B.M.; Smith, J.M.; Jee, W.S. Characterization of endosteal bone-lining cells from fatty marrow bone sites in adult beagles. Anat. Rec. 1980, 198, 163–173.
- Doty, S.B. Cell-to-cell communication in bone tissue. In Davidovich V: The Biological Mechanism of Tooth Eruption and Root Resorption; Davidovitch, V., Ed.; EBSCO Media: Birmingham, AL, USA, 1988; pp. 61–69.
- Sakata, T.; Sakai, A.; Tsurukami, H.; Okimoto, N.; Okazaki, Y.; Ikeda, S.; Norimura, T.; Nakamura, T. Trabecular bone turnover and bone marrow cell development in tail-suspended mice. J. Bone Miner. Res. 1999, 14, 1596–1604.
- Bikle, D.D.; Sakata, T.; Halloran, B.P. The impact of skeletal unloading on bone formation. Gravit. Space Biol. Bull. 2003, 16, 45–54.
- Kerstetter, J.E.; O’Brien, K.O.; Insogna, K.L. Dietary protein, calcium metabolism, and skeletal homeostasis revisited. Am. J. Clin. Nutr. 2003, 78 (Suppl. S3), S584–S592.
- Ferretti, M.; Cavani, F.; Smargiassi, A.; Roli, L.; Palumbo, C. Mineral and skeletal homeostasis influence the manner of bone loss in metabolic osteoporosis due to calcium-deprived diet in different sites of rat vertebra and femur. BioMed Res. Int. 2015, 304178.
- Ferretti, M.; Cavani, F.; Roli, L.; Checchi, M.; Magarò, M.S.; Bertacchini, J.; Palumbo, C. Interaction among calcium diet content, PTH(1-34) treatment and balance of bone homeostasis in rat model: The trabecular bone as keystone. Int. J. Mol. Sci. 2019, 20, 753.
- Lewiecki, E.M. Romosozumab, clinical trials, and real-world care of patients with osteoporosis. Ann. Transl. Med. 2020, 8, 974.
- Skripitz, R.; Andreassen, T.T.; Aspenberg, P. Strong effect of PTH (1-34) on regenerating bone: A time sequence study in rats. Acta Orthop. Scand. 2000, 71, 619–624.
- Orwoll, E.S.; Scheele, W.H.; Paul, S.; Adami, S.; Syversen, U.; Diez-Perez, A.; Kaufman, J.M.; Clancy, A.D.; Gaich, G.A. The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J. Bone Miner. Res. 2003, 18, 9–17.
- Lindsay, R.; Zhou, H.; Cosman, F.; Nieves, J.; Dempster, D.W.; Hodsman, A.B. Effects of a one-month treatment with PTH (1-34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J. Bone Miner. Res. 2007, 22, 495–502.
- Tanaka, S.; Kuroda, T.; Sugimoto, T.; Nakamura, T.; Shiraki, M. Changes in bone mineral density, bone turnover markers, and vertebral fracture risk reduction with once weekly teriparatide. Curr. Med. Res. Opin. 2014, 30, 931–936.
- Hasegawa, T.; Amizuka, N. Bone remodeling and modeling/mini-modeling. Clin. Calcium 2017, 27, 1713–1722.
- Kumabe, Y.; Lee, S.Y.; Waki, T.; Iwakura, T.; Takahara, S.; Arakura, M.; Kuroiwa, Y.; Fukui, T.; Matsumoto, T.; Matsushita, T.; et al. 3Triweekly administration of parathyroid hormone (1–34) accelerates bone healing in a rat refractory fracture model. BMC Musculoskelet. Disord. 2017, 18, 545.
- Canalis, E. Management of endocrine disease: Novel anabolic treatments for osteoporosis. Eur. J. Endocrinol. 2018, 178, R33–R44.
- Dehority, W.; Halloran, B.P.; Bikle, D.D.; Curren, T.; Kostenuik, P.J.; Wronski, T.J.; Shen, Y.; Rabkin, B.; Bouraoui, A.; Morey-Holton, E. Bone and hormonal changes induced by skeletal unloading in the mature male rat. Am. J. Physiol. 1999, 276P, E62–E69.
- Iwamoto, J.; Yeh, J.K.; Aloia, J.F. Differential effect of treadmill exercise on three cancellous bone sites in the young growing rat. Bone 1999, 24, 163–169.
- Lecoq, B.; Potrel-Burgot, C.; Granier, P.; Sabatier, J.P.; Marcelli, C. Comparison of bone loss induced in female rats by hindlimb unloading, ovariectomy, or both. Jt. Bone Spine 2006, 73, 189–195.
- Burgers, T.A.; Williams, B.O. Regulation of Wnt/β-catenin signaling within and from osteocytes. Bone 2013, 54, 244–249.
