Nutritional management is one of the most important factors to ensure adequate productivity and to prevent wasting in sheep flocks.
- sheep
- nutritional management
1. Introduction
Sheep are reared in a broad variety of geographical locations and production systems across the world. Sheep flocks may have different purposes, including meat, milk, and wool production, or, most frequently, a combination of two or three of them [1]. Irrespective of the breeding purpose of a flock, maintaining a good body condition is a basic pillar of the nutritional and health management in order to maximize productivity [2].
Many diseases may compromise sheep body condition, affecting either directly or indirectly food intake and/or nutrient assimilation. Perhaps the most well-known amongst these processes are those related to infectious and parasitic agents [3[3][4][5][6][7],4,5,6,7], which over the years have generated many research and diagnostic efforts to prevent their harmful effects. There are also several nutritional, metabolic, and digestive motility disorders that may disrupt nutrient assimilation and cause loss of condition. A correctly managed diet, which ensures that enough quality food is offered to the animals, is key to prevent wasting due to starving and/or imbalanced mineral intake.
2. Malnutrition
2. Malnutrition
An adequate diet is the first requirement that needs to be fulfilled in order to prevent states of wasting in sheep. Feed needs may vary depending upon the purpose of the flock but, in general, supplementation is needed in periods of scarcity, such as winter months [8]. This is especially relevant in purely extensive systems. Malnutrition has a prominent impact on the reproductive function [9], and thus the pre-mating body condition is one of the most important parameters to be controlled in the majority of systems [10].
Emaciation in animals may be the result of two different mechanisms: cachexia, which is cytokine-mediated and associated with endogenous disease; and starvation, which is due to a reduction in the caloric intake and generally related to exogenous circumstances (e.g., food deprivation and/or scarcity, adverse environmental or management conditions, etc.) [11,12]. Unfortunately, there is no definitive parameter available to differentiate emaciation due to exogenous circumstances (starvation) from cachexia, and a good history focused on the dietary and health management of the flock should be the first diagnostic approach to take. Then, if deemed necessary, multiple diagnostic tests that rule out endogenous disease may be applied [12].
Body condition assessment is valuable to determine the nutritional status of sheep. Animals are palpated on the loin, immediately behind the last rib, and the prominence of the spinous and transverse vertebral processes is evaluated subjectively by estimating the amount of muscle (
An adequate diet is the first requirement that needs to be fulfilled in order to prevent states of wasting in sheep. Feed needs may vary depending upon the purpose of the flock but, in general, supplementation is needed in periods of scarcity, such as winter months [8]. This is especially relevant in purely extensive systems. Malnutrition has a prominent impact on the reproductive function [9], and thus the pre-mating body condition is one of the most important parameters to be controlled in the majority of systems [10].
Emaciation in animals may be the result of two different mechanisms: cachexia, which is cytokine-mediated and associated with endogenous disease; and starvation, which is due to a reduction in the caloric intake and generally related to exogenous circumstances (e.g., food deprivation and/or scarcity, adverse environmental or management conditions, etc.) [11][12]. Unfortunately, there is no definitive parameter available to differentiate emaciation due to exogenous circumstances (starvation) from cachexia, and a good history focused on the dietary and health management of the flock should be the first diagnostic approach to take. Then, if deemed necessary, multiple diagnostic tests that rule out endogenous disease may be applied [12].
Body condition assessment is valuable to determine the nutritional status of sheep. Animals are palpated on the loin, immediately behind the last rib, and the prominence of the spinous and transverse vertebral processes is evaluated subjectively by estimating the amount of muscle (
longissimus dorsi) and fat covering these prominences. A six-tier system is commonly used, 0 being totally emaciated and 5 being obese [13]. An adequate body condition score should range between 2 and 4, depending upon the purpose of the flock, breeding stage, and geographical location [10].
The postmortem examination of an emaciated sheep should be focused on ruling out lesions associated with endogenous causes of disease, e.g., Johne’s disease, maedi-visna, endoparasitosis, poor dentition, etc. [3,4,6,14,15], that may have prompted such status. Grossly, a sheep that died from chronic starvation usually presents with depletion and serous atrophy of fat deposits. Serous atrophy (also known as gelatinous transformation or myxomatous degeneration) occurs as a result of mobilization of fat reserves, which leads to a watery to jelly-like consistency of the remaining adipose tissue deposits [14,16,17]. Affected regions usually include epicardial, pericardial, perirenal, mesenteric, omental, and bone marrow fat deposits [14,18]. Effusions in body cavities, including pericardial sac, abdomen, and thorax, as well as a degree of subcutaneous edema, may also be detected and are likely due to hypoproteinemia. In extreme cases, muscle atrophy ensues, and the liver may appear small, likely due to loss of trophic stimuli [18]. Microscopic examination may help to demonstrate endogenous causes of emaciation that were missed grossly, but generally does not add much information in cases of starvation. Atrophic adipocytes over a faintly basophilic to amphophilic, extracellular matrix are detected in the depleted fat stores. This matrix stains light blue with Alcian blue, and is thus believed to be made of mucopolysaccharides rich in hyaluronic acid [19].
Measuring the percentage of fat in the bone marrow of a long bone by quantitative methods can provide with an unbiased evidence of adipose tissue depletion [20,21]. The average percentage of fat in the femoral bone marrow for animals in good body condition is >80%, whereas emaciated individuals present with values of less than 20% [20]. Vitamin and mineral imbalances may occur concomitantly in chronically emaciated sheep, thus evaluating their levels adds information to the clinico-pathologic picture [22]. In addition, undernourished sheep are predisposed to parasitosis by gastrointestinal nematodes [17,23], which may contribute further to loss of condition. Sheep tend to respond well to the reestablishment of an adequate diet, thus correction of the deficient management procedures is the main therapeutic approach in flocks with malnutrition [22].
3. Subacute and Chronic Ruminal Acidosis
) and fat covering these prominences. A six-tier system is commonly used, 0 being totally emaciated and 5 being obese [13]. An adequate body condition score should range between 2 and 4, depending upon the purpose of the flock, breeding stage, and geographical location [10].
The postmortem examination of an emaciated sheep should be focused on ruling out lesions associated with endogenous causes of disease, e.g., Johne’s disease, maedi-visna, endoparasitosis, poor dentition, etc. [3][4][6][14][15], that may have prompted such status. Grossly, a sheep that died from chronic starvation usually presents with depletion and serous atrophy of fat deposits. Serous atrophy (also known as gelatinous transformation or myxomatous degeneration) occurs as a result of mobilization of fat reserves, which leads to a watery to jelly-like consistency of the remaining adipose tissue deposits [14][16][17]. Affected regions usually include epicardial, pericardial, perirenal, mesenteric, omental, and bone marrow fat deposits [14][18]. Effusions in body cavities, including pericardial sac, abdomen, and thorax, as well as a degree of subcutaneous edema, may also be detected and are likely due to hypoproteinemia. In extreme cases, muscle atrophy ensues, and the liver may appear small, likely due to loss of trophic stimuli [18]. Microscopic examination may help to demonstrate endogenous causes of emaciation that were missed grossly, but generally does not add much information in cases of starvation. Atrophic adipocytes over a faintly basophilic to amphophilic, extracellular matrix are detected in the depleted fat stores. This matrix stains light blue with Alcian blue, and is thus believed to be made of mucopolysaccharides rich in hyaluronic acid [19].
Measuring the percentage of fat in the bone marrow of a long bone by quantitative methods can provide with an unbiased evidence of adipose tissue depletion [20][21]. The average percentage of fat in the femoral bone marrow for animals in good body condition is >80%, whereas emaciated individuals present with values of less than 20% [20]. Vitamin and mineral imbalances may occur concomitantly in chronically emaciated sheep, thus evaluating their levels adds information to the clinico-pathologic picture [22]. In addition, undernourished sheep are predisposed to parasitosis by gastrointestinal nematodes [17][23], which may contribute further to loss of condition. Sheep tend to respond well to the reestablishment of an adequate diet, thus correction of the deficient management procedures is the main therapeutic approach in flocks with malnutrition [22].
3. Subacute and Chronic Ruminal Acidosis
Rumen acidosis (ruminal acidosis, rumen lactic acidosis, grain overload, rumenitis) is a metabolic disorder that occurs after the ingestion of readily fermentable carbohydrates and their subsequent rapid ruminal fermentation [24]. The disease occurs frequently in cattle and small ruminants [18[18][24][25],24,25], and may be acute, sub-acute, or chronic [25].
Ruminal acidosis associated with ingestion of excess carbohydrate in sheep is usually associated with intensive breeding systems [18,25][18][25]. While acute acidosis usually causes death, sub-acute and chronic acidosis are responsible for loss of production. Sub-acute and chronic acidosis cause rumenitis, which is also important as a port of entry for Fusobacterium necrophorum and fungi, thus leading to secondary ruminal and hepatic infections. Primary tympany (frothy bloat) is another complication of rumenitis [18].
The pathogenesis of acute ruminal acidosis is usually the consequence of sudden ingestion of excess carbohydrate in animals not accustomed to it (Figure 1). These carbohydrates include, amongst others, grain, bread, waste baked goods, brewers’ waste, root crops, and apples. The amount of carbohydrate necessary to produce acidosis is highly variable and the individual tolerance increases when those carbohydrates are introduced progressively. Hence, the sudden introduction or increase of carbohydrates are more critical than the actual amount [18,25][18][25].
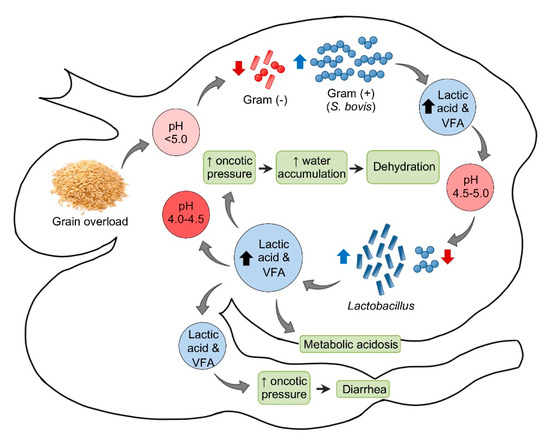
Pathogenesis of ruminal acidosis. VFA = Volatile fatty acids.
In sheep, the normal ruminal pH is 5.5–7.5, depending on the diet, with Gram-negative bacteria being the predominant component of the ruminal microbiota. Very soon after consumption of a large amount of carbohydrate to an unaccustomed animal, the ruminal pH begins to drop. This initial decrease in pH is mainly the consequence of an increase in dissociated volatile fatty acids (VFA). Most Gram-negative bacteria and protozoa, which are very sensitive to changes in pH, die when the pH reaches 5.0 or below. When this happens, Streptococcus bovis and other streptococci start to proliferate rapidly and produce lactic acid, which reduces the pH to 5.0–4.5. At this point, there is a switch in the ruminal microbiota, characterized by an increase in lactobacilli and a decrease in streptococci. In fatal cases, the pH of the rumen content may fall as low as 4.0–4.5 [18].
Saliva secretion also stops, which eliminates the important buffering effect of this substance. As the concentrations of the non-dissociated VFA, lactic, propionic, and butyric increase, these acids act on receptors that mediate inhibition of reticuloruminal motility via a vagovagal reflex, leading to ruminal atony. The ruminal oncotic pressure increases because of the increased concentration of these ruminal organic acids, mainly lactate. The consequence of this is accumulation of water in the rumen and severe dehydration with reduction of plasma volume, hemoconcentration, anuria, and circulatory collapse. When lactate passes through the intestine, the oncotic pressure of the intestinal content also increases producing liquid content in this part of the intestinal tract and thus leading to diarrhea. Lactate is also absorbed from the rumen, and possibly from the intestine, leading to metabolic acidosis. As a consequence of general dehydration, there is an increase of serum protein, urea, inorganic phosphorus, lactate, pyruvate, and liver enzymes. Most of the normal ruminal flora and fauna die as a result of the low pH. Animals that survive acute ruminal acidosis need to have their ruminal microbiota re-established. This may occur by therapeutic ruminal content transplant or by contact with feces of unaffected animals [18]. Sub-acute and chronic acidosis have a similar pathogenesis, although the drop in pH tends to be milder and persistent, leading to physical damage of the ruminal mucosa and submucosa.
Clinical signs of acute ruminal acidosis may be observed within 12 h after ingestion of concentrate, and they include lethargy, anorexia, bruxism, nasal discharge, head pressing, ataxia, hyperpnea, recumbency, dehydration, scleral injection, muscle twitching, tachycardia, fluid abdominal sounds, reduced or absent motility of the rumen, mild colic, diarrhea and pH reduction to 5 or 6. In severe cases, shock and coma leading to death may also occur [24,26][24][26].
In subacute and chronic ruminal acidosis, clinical signs include reduced or cyclic feed intake, decreased milk production, reduced fat, poor body condition and diarrhea. Additionally, unusually high rates of culling or unexplained deaths may be noted in the flock. Diarrhea is inconsistently seen [27]. Laminitis is a traditionally suggested sequel of all forms of rumen acidosis [24,25][24][25]; however, the pathogenesis for acidosis and laminitis has been recently disputed in cattle, since there seems to be lack of clear evidence to support the classic laminitis hypothesis [25,28][25][28]. Further research into the mechanistic basis of the association between ruminal acidosis and laminitis in sheep is necessary.
The gross findings of acute ruminal acidosis are not specific and are characterized by signs of dehydration and hypoxia, including sunken eyes, dense and dark blood, and general venous congestion. Soon after the ingestion of large amounts of carbohydrates, the rumen content has a porridge-like appearance with a distinct fermentative odor. There may or may not be a large amount of grain or other sources of starch, but care should be taken not to overlook finely ground concentrate. In sub-acute or chronic cases of acidosis, the ruminal content may appear more or less normal, but the intestinal contents remain watery. There may be a poorly defined slight blue discoloration in the ventral sac of the rumen and reticulum and in the omasum, visible through the serosa. When the epithelium is detached, the lamina propria is patchy hyperemic. In some cases, the epithelium appears to have undergone fixation due to low pH, and is difficult to peel [18]. Patches of flat, white ruminal mucosa devoid of papillae, and multiple abscesses or foci of necrosis may be evident in the liver. Interpretation of the post-mortem rumen pH may be challenging. Some authors consider that because ruminal fermentation continues after death, the pH of rumen contents declines postmortem [29].
Microscopic changes are usually absent in the rumen of animals dying of acute rumen acidosis, unless pre-existing subacute lesions were present before the onset of acute disease. Microscopic examination of the rumen in cases of sub-acute or chronic acidosis reveals lesions consistent with chemical rumenitis, including enlarged ruminal papillae, cytoplasmic vacuolation of the epithelial cells often leading to vesiculation, and a mild to marked neutrophilic infiltration in the mucosa and submucosa (Figure 2). There may be multifocal erosion and ulceration of the superficial mucosa. Absence of protozoa is consistent with chemical rumenitis, but is also influenced by the interval between death and the postmortem examination. Similar changes may be detected in the other pre-stomachs. Apart from general congestion, there are no specific changes in other organs [18].
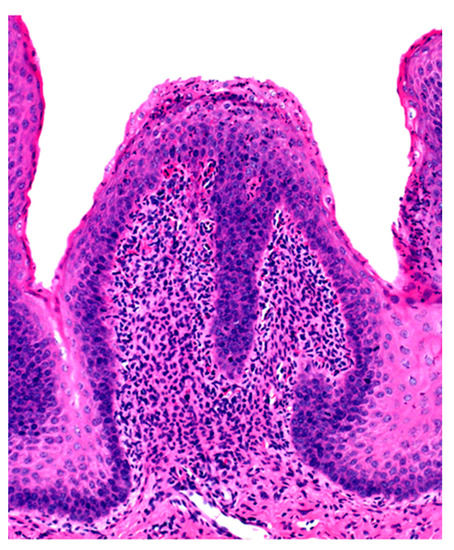
Ruminal papilla with intraepithelial neutrophilic aggregates and pleocellular inflammation in the lamina propria.
References
- Morris, S.T. Overview of sheep production systems. In Advances in Sheep Welfare, 1st ed.; Ferguson, D.M., Lee, C., Fisher, A., Eds.; Woodhead Publishing: Kidlington, UK, 2017; pp. 19–35.
- Lovatt, F. Developing flock health plans. Practice 2004, 26, 290–295.
- Zajac, A.M. Gastrointestinal nematodes of small ruminants: Life cycle, anthelmintics, and diagnosis. Vet. Clin. N. Am. Food Anim. Pract. 2006, 22, 529–541.
- Baird, G.J.; Fontaine, M.C. Corynebacterium pseudotuberculosis and its role in ovine caseous lymphadenitis. J. Comp. Pathol. 2007, 137, 179–210.
- Windsor, P.A. Paratuberculosis in sheep and goats. Vet. Microbiol. 2015, 181, 161–169.
- Minguijón, E.; Reina, R.; Pérez, M.; Polledo, L.; Villoria, M.; Ramírez, H.; Leginagoikoa, I.; Badiola, J.J.; García-Marín, J.F.; de Andrés, D.; et al. Small ruminant lentivirus infections and diseases. Vet. Microbiol. 2015, 181, 75–89.
- Windsor, P.; Whittington, R. Ovine Paratuberculosis Control in Australia Revisited. Animals 2020, 10, 1623.
- Winter, A.C.; Fitzpatrick, J.L. Sheep Welfare: Standards and Practices. In Diseases of Sheep, 4th ed.; Aitken, I.D., Ed.; Blackwell Publishing: Oxford, UK, 2007; pp. 15–22.
- Abecia, J.A.; Sosa, C.; Forcada, F.; Meikle, A. The effect of undernutrition on the establishment of pregnancy in the ewe. Reprod. Nutr. Dev. 2006, 46, 367–378.
- Stubbings, L.A. Ewe Management for Reproduction. In Diseases of Sheep, 4th ed.; Aitken, I.D., Ed.; Blackwell Publishing: Oxford, UK, 2007; pp. 53–61.
- Morley, J.E.; Thomas, D.R.; Wilson, M.M. Cachexia: Pathophysiology and clinical relevance. Am. J. Clin. Nutr. 2006, 83, 735–743.
- Gerdin, J.A.; McDonough, S.P.; Reisman, R.; Scarlett, J. Circumstances, Descriptive Characteristics, and Pathologic Findings in Dogs Suspected of Starving. Vet. Pathol. 2016, 53, 1087–1094.
- Russel, A. Body condition scoring of sheep. Practice 1984, 6, 91–93.
- Bush, R.D.; Toribio, J.A.; Windsor, P.A. The impact of malnutrition and other causes of losses of adult sheep in 12 flocks during drought. Aust. Vet. J. 2006, 84, 254–260.
- Ruiz de Arcaute Rivero, M.; Ferrer-Mayayo, L.-M.; Lacasta, D.; González, J.; Las Heras, M.; Borobia, M.; Ramos, J.J. Prevalence of dental and mandibular disorders in culled sheep in Spain. Aust. Vet. J. 2020, 98, 438–441.
- Whiting, T.L.; Postey, R.C.; Chestley, S.T.; Wruck, G.C. Explanatory model of cattle death by starvation in Manitoba: Forensic evaluation. Can. Vet. J. 2012, 53, 1173–1180.
- Inadequate feeding and grazing leads to illthrift and death in cattle and sheep. Vet. Rec. 2018, 183, 51.
- Uzal, F.A.; Plattner, B.L.; Hostetter, J.M. Alimentary System. In Jubb, Kennedy & Palmer’s Pathology of Domestic Animals: Volume 2, 6th ed.; Maxie, M.G., Ed.; W.B. Saunders: St. Louis, MO, USA, 2016; pp. 1–257.
- Beeler-Marfisi, J.; Gallastegui Menoyo, A.; Beck, A.; König, J.; Hewson, J.; Bienzle, D. Gelatinous marrow transformation and hematopoietic atrophy in a miniature horse stallion. Vet. Pathol. 2011, 48, 451–455.
- Meyerholtz, K.A.; Wilson, C.R.; Everson, R.J.; Hooser, S.B. Quantitative assessment of the percent fat in domestic animal bone marrow. J. Forensic Sci. 2011, 56, 775–777.
- Murden, D.; Hunnam, J.; De Groef, B.; Rawlin, G.; McCowan, C. Comparison of methodologies in determining bone marrow fat percentage under different environmental conditions. J. Vet. Diagn. Investig. 2017, 29, 83–90.
- Sherman, D.M. Unexplained weight loss in sheep and goats. A guide to differential diagnosis, therapy, and management. Vet. Clin. N. Am. Large Anim. Pract. 1983, 5, 571–590.
- Coop, R.L.; Holmes, P.H. Nutrition and parasite interaction. Int. J. Parasitol. 1996, 26, 951–962.
- Van Metre, D.C.; Tyler, J.W.; Stehman, S.M. Diagnosis of enteric disease in small ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 87–115.
- Kleen, J.L.; Hooijer, G.A.; Rehage, J.; Noordhuizen, J.P. Subacute ruminal acidosis (SARA): A review. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2003, 50, 406–414.
- Braun, U.; Rihs, T.; Schefer, U. Ruminal lactic acidosis in sheep and goats. Vet. Rec. 1992, 130, 343–349.
- Lorentz, I. Subacute Ruminal Acidosis. In Merck Veterinary Manual. 2015. Available online: (accessed on 29 November 2020).
- Randall, L.V.; Green, M.J.; Huxley, J.N. Use of statistical modelling to investigate the pathogenesis of claw horn disruption lesions in dairy cattle. Vet. J. 2018, 238, 41–48.
- Cole, N.; Richardson, L.; Stock, R. Postmortem ruminal changes in sheep and steers. Vet. Clin. Nutr. 1998, 5, 14–17.
