Ionic liquids (ILs) are molten salts composed of a large organic cation and an organic/inorganic anion. The large dimensions of their ions lead to charge dispersion, which makes difficult the formation of a regular crystalline structure. Due to their unique properties, ILs have been applied in the crystallization of active pharmaceutical ingredients (APIs), as solvents, co-solvents and emulsifiers in drug formulations, as pharmaceuticals (API-ILs) aiming liquid therapeutics, and in the development and/or improvement of drug-delivery-based systems.
- Active pharmaceutical ingredients
- Drug delivery
- Ionic liquids
1. Introduction
Pharmaceuticals play a major role in medical care, boosting life quality and expectancy, especially when considering chronic diseases [1]. The global prescription of medicines is forecast to grow up to nearly $1.2 trillion by 2022 [2]. Although active pharmaceutical ingredients (APIs) can be commercialized in several dosage forms, crystalline forms have been the preferred option [3][4]. However, 40 to 70% of the drugs under development present low water-solubility, which may compromise the bioavailability and therapeutic efficacy and, thus, fail in the later stages of development [5][6]. The irregular gastrointestinal absorption of solid forms, along with the low therapeutic efficiency and possible toxicity and side-effects of polymorphs, are major concerns to overcome [7]. For instance, large differences in bioavailability among different polymorphs require different drug dosages [8]. On the other hand, the therapeutic dosage of a certain API can correspond to a toxic or potential lethal dose if the wrong polymorph is administered. Polymorphism issues result in significant economic losses in sales and in R&D to enable novel formulations back into the market [9][10].
Beyond the well-known downsides of polymorphism, the APIs’ solubility in aqueous solution, dissolution, and bioavailability are also dependent on particle size and properties [11]. Attempting to improve the drugs solubility in water as well as their bioavailability, several strategies have been investigated, especially when the oral route is envisaged [5][6]. Nevertheless, most of these strategies still use large quantities of organic solvents in the manufacturing process of these formulations, particularly to induce the crystallization of a given polymorphic form and particle size, having associated health and environmental concerns [12]. Furthermore, solvent molecules can be incorporated into the crystal structure of the API during the crystallization process [13]. Therefore, when considering the use of organic solvents, they must be removed from the API or their levels must be controlled in order to ensure human consumption safety [12]. Despite the existence of extensive literature describing novel and “greener” solvents to this purpose, there is still some reluctance by the pharmaceutical industry to accept and implement these alternatives [14][15][16].
In the above context, liquid forms of APIs are appealing solutions to avoid both polymorphism and improve low-water solubility constraints, while allowing to reduce organic solvents use. The pharmaceutical industry has relied on eutectic mixtures for this purpose, shortly exploring other options for commercialization [17][18]. In addition to these, ionic liquids (ILs) disclose high potential in the pharmaceutical field, which is mainly due to their high versatility in terms of chemical structure design towards a target application. ILs are molten salts that are composed of a large organic cation and an organic/inorganic anion. The large dimensions of their ions lead to charge dispersion, which makes difficult the formation of a regular crystalline structure [19][20]. ILs display a set of unique features, from which is possible to highlight, if properly designed, their high thermal and chemical stability and a strong solvation ability for a wide variety of compounds [21]. The proper selection of cation-anion combinations in ILs enables the use of drugs as ion components, allowing for the conversion of solid active pharmaceutical ingredients into liquid forms (API-ILs). Thus, this strategy solves the problem of polymorphism and provides improved bioavailability, and ideally boosts therapeutic properties [3][22].
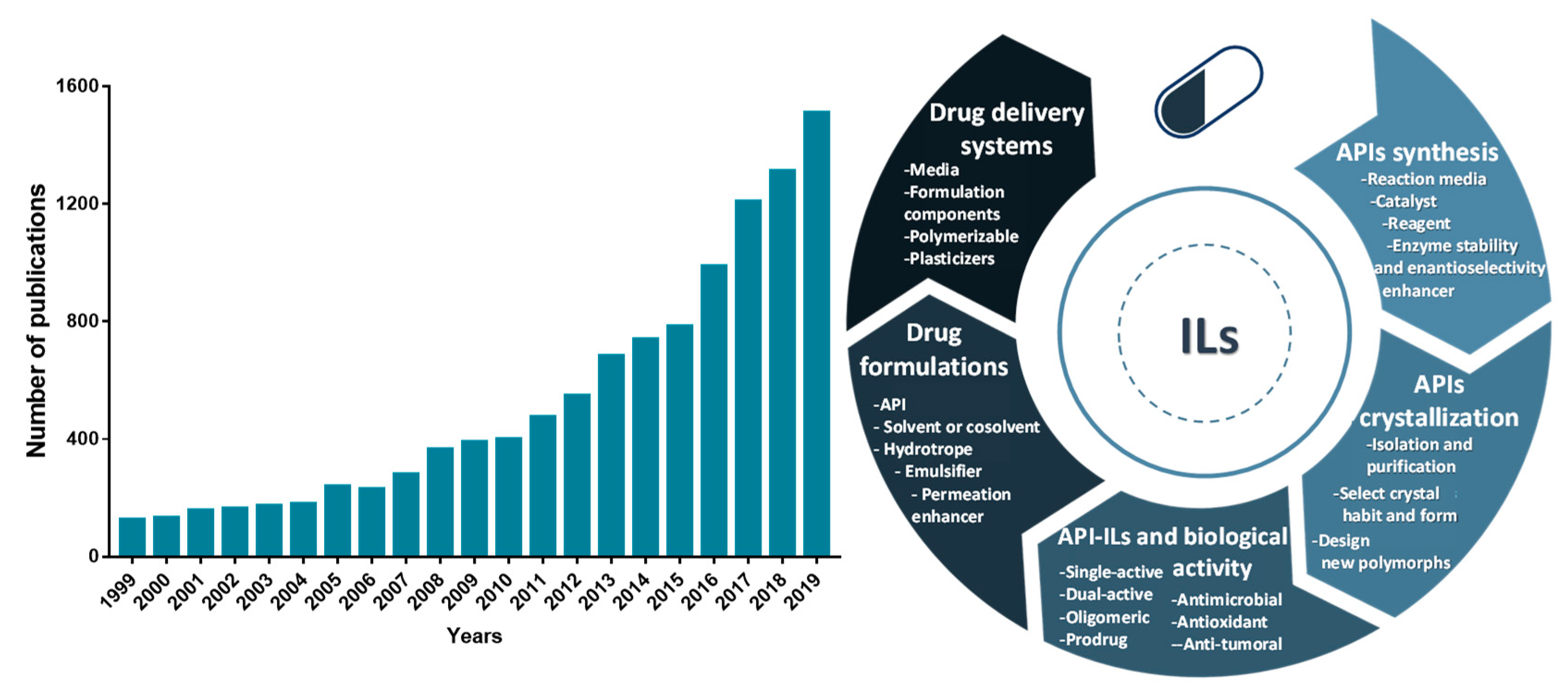
Because of the unique properties of ILs, their application in the pharmaceutical field has been extended far beyond the development of novel liquid forms (API-ILs), being investigated as well in other stages of drug development and delivery. The number of publications related to the application of ILs in the pharmaceutical field has grown exponentially in the past 20 years, as illustrated in Figure 1. ILs have been applied in the development of purification platforms for pharmaceuticals for which some recent review manuscripts exist [23][24][25]. Other relevant reviews and book chapters recognizing the advances of ILs in different areas of pharmaceuticals development, spanning from their formulation, biological activity, and application on drug delivery are also available [22][26][27][28][29][30][31][32][33][34].
Figure 1. Number of publications per year in a twenty years perspective related to ILs and active pharmaceutical ingredients (APIs) (number of articles, reviews and book chapters according to a ScienceDirect database search using as keywords “ionic liquids”, “active pharmaceutical ingredients”, and “drug delivery”) (left). Overview of the ILs’ applications in the pharmaceutical field reported hitherto (right).
2. The Role of Ionic Liquids in the Pharmaceutical Field
Organic volatile solvents have been a main choice in the pharmaceutical industry, particularly in the reaction and purification steps, but they still raise concerns on the contamination of the final product and on the related environmental and health impacts. To overcome some of these drawbacks, ILs have been investigated as solvents, reagents, or catalysts in the synthesis of APIs and applied in the crystallization process of drugs. Still, additional studies should focus on the development of more sustainable strategies to remove ILs after the APIs synthesis. A careful monitorization of these contaminants in the final product and study of their impact in the drug’s performance and toxicity must be taking into consideration. Furthermore, the use of solvents and co-solvents, like ethanol, methanol or DMSO, hydrotropes, and surface-active agents, to improve the API’s solubility in pharmaceutical formulations has been challenged by applying ILs to this purpose. ILs have been shown to allow the aqueous solubility improvement of APIs from distinct pharmacological classes in several orders of magnitude (to be best of our knowledge and up to date, up to 5.6 × 106-fold), standing as competitive alternatives to organic solvents. However, these formulations require a more comprehensive study in what concerns the stability, absorption and bioavailability of APIs. Furthermore, more recent studies employing aqueous solutions of ILs instead of pure ILs can be the key for their use and acceptance by the pharmaceutical field.
Because the APIs’ solubility in aqueous solution and bioavailability can be limited by polymorphism, controlling this process is essential for obtaining a stable and high-quality drug product. The study of ILs in the crystallization process of APIs has enabled the possibility to design new polymorphs, with higher thermal stability, to select the crystal form and habit, and even isolate and purify the correct API polymorph through crystallization. To expand the research on this topic, it is still mandatory to comprehensively understand the IL-API interactions that drive the formation of specific polymorphic forms and habits. The use of computational tools can be helpful in designing ILs in such a way. Furthermore, as it happens with the APIs synthesis, the research on effective separation methods and the limitation of the IL contamination in the final product are highly demanding issues.
ILs can be designed to present biological activities due to the broad number of anion-cation combinations. In this sense, ILs have been successfully studied regarding their antimicrobial, antioxidant, and anti-tumoral activities. So far, IL activities have been mainly studied in vitro and have focused on imidazolium-based ILs. To expand this field of research, it is necessary to unveil the mechanisms of interaction between the IL and biological membranes and, consequently, establish a correlation with their biological activities, and in which computational tools may also play a crucial role. The possibility to manipulate the cation-anion combinations also allowed for obtaining new drugs with desired chemical and biological properties, while avoiding polymorphism concerns. API-ILs have provided double action in therapeutic formulations for topical and transdermal delivery, namely by providing the API facilitating its permeation through biological membranes. In this field, API-ILs stand as novel liquid forms that can be designed with a specific or dual pharmacological action, obtained by incorporating APIs as IL ions, using oligomeric ions or by applying a prodrug strategy. However, only few works conduct bioavailability assays and allow to demonstrate the increase in the API’s bioavailability and the therapeutic efficacy of these novel drugs. Because API-ILs can present contrastive, enhanced or even dual effect when compared to the initial precursors, in vivo pharmacokinetic and pharmacodynamic tests are mandatory to understand the pathways that are involved in their absorption, metabolism, and routes of elimination. These assays are required to enable the acceptance of these new drugs by the pharmaceutical industry, favoring the establishment of guidelines for their development and research. Until their implementation, additional obstacles are expected to be faced. The pharmaceutical industry is majorly prepared in order to produce solid APIs; thus, the scale-up implementation might be a lengthy process. Years of routinely working with solid forms of APIs might make the development of standardized procedures for the liquid drugs’ purification difficult.
The flexibility of ILs allows the development of tailored (bio)polymer drug delivery systems as well, both due to their polymerizable character and polymer solvation ability. Because ILs can enhance the APIs solubility in aqueous media, they have successfully allowed the incorporation and delivery of several low-water soluble drugs, enabling the consideration of new administration routes. However, the lack of more complete studies on this topic that can assist the conscious development of more effective drug delivery options still confines their use and commercialization. Advances in this area should comprise integrated studies where the IL can be designed with a specific biological activity and/or therapeutic action. These designed ILs can simultaneously have a specific role in the development of the drug delivery systems. In this line, stimuli-responsive drug delivery systems, promoted by the IL and/or by the polymer, also are of particular relevance.
Overall, ILs have potential to overcome solubility, bioavailability, permeation, polymorphism, and stability concerns that are associated to solid-state pharmaceuticals. Furthermore, ILs are promising alternatives to volatile organic solvents when applied as solvents, reagents, and anti-solvents in the synthesis and crystallization of APIs. More recent studies demonstrate their potential to improve the performance of drug-delivery-based systems. The results and advances reported so far support the multiple roles of ILs in the pharmaceutical field, encouraging new ways of taking advantage of their unique properties.
References
- World Health Organization Medicines Strategy-Contries at the Core. Available online: https://apps.who.int/iris/bitstream/handle/10665/84307/WHO_EDM_2004.5_eng.pdf;jsessionid=0F733DD987692B73A234E9FB8C10D40B?sequence=1 (accessed on 20 May 2020).
- Ende, M.T.; Ende, D.J. Chemical Engineering in the Pharmaceutical Industry: Drug Product Design, Development and Modeling, 2nd ed.; Wiley: Hoboken, NJ, USA, 2019.
- Shamshina, J.L.; Rogers, R.D. Overcoming the problems of solid state drug formulations with ionic liquids. Ther. Deliv. 2014, 5, 489–491.
- Byrn, S.; Pfeiffer, R.; Ganey, M.; Hoiber, C.; Poochikian, G. Pharmaceutical Solids: A Strategic Approach to Regulatory Considerations. Pharm. Res. 1995, 12, 945–954.
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453.
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727.
- Brittain, H.G.; Grant, D.J.R. Effects of Polymorphism and Solid-State Solvation on Solubility and Dissolution Rate. In Polymorphism in Pharmaceutical Solids; Taylor and Francis: Abingdon, UK, 2009; pp. 436–480.
- Censi, R.; Di Martino, P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 2015, 20, 18759–18776.
- Bauer, J.; Spanton, S.; Henry, R.; Quick, J.; Dziki, W.; Porter, W.; Morris, J. Ritonavir: An Extraordinary Case of Conformational Polymorphism. Pharm. Res. 2001, 18, 859–866.
- Hulme, A.T.; Price, S.L.; Tocher, D.A. A New Polymorph of 5-Fluorouracil Found Following Computational Crystal Structure Predictions. J. Am. Chem. Soc. 2005, 127, 1116–1117.
- Florence, A.T.; Attwood, D. Physicochemical Principles of Pharmacy, 4th ed.; Pharmaceutical Press: London, UK, 2006.
- Cue, B.W.; Zhang, J. Green process chemistry in the pharmaceutical industry. Green Chem. Lett. Rev. 2009, 2, 193–211.
- El-Yafi, A.K.E.Z.; El-Zein, H. Technical crystallization for application in pharmaceutical material engineering: Review article. Asian J. Pharm. Sci. 2015, 10, 283–291.
- Jain, N.; Kumar, A.; Chauhan, S.; Chauhan, S.M.S. Chemical and biochemical transformations in ionic liquids. Tetrahedron 2005, 61, 1015–1060.
- Olivier-bourbigou, H.; Magna, L. Ionic liquids: Perspectives for organic and catalytic reactions. J. Mol. Catal. A Chem. 2002, 183, 419–437.
- Cave, G.W.V.; Raston, L.; Scott, J.L. Recent advances in solventless organic reactions: Towards benign synthesis with remarkable versatility. Chem. Commun. 2001, 2001, 2159–2169.
- Ehrenström-Reiz, G.; Reiz, S.; Stockman, O. Topical Anaesthesia with EMLA, a New Lidocaine-Prilocaine Cream and the Cusum Technique for Detection of Minimal Application Time. Acta Anaesthesiol. Scand. 1983, 27, 510–512.
- Shamshina, J.L.; Kelley, S.P.; Gurau, G.; Rogers, R.D. Develop ionic liquid drugs. Nature 2015, 528, 188–189.
- Freire, M.G.; Cláudio, A.F.M.; Araújo, J.M.M.; Coutinho, J.A.P.; Marrucho, I.M.; Canongia Lopes, J.N.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995.
- Earle, M.J.; Esperança, J.M.S.S.; Gile, M.; Lopes, J.N.C.; Rebelo, L.P.N.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The distillation and volatility of ionic liquids. Nature 2005, 439, 831–834.
- Cláudio, A.F.M.; Neves, M.C.; Shimizu, K.; Canongia Lopes, J.N.; Freire, M.G.; Coutinho, J.A.P. The magic of aqueous solutions of ionic liquids: Ionic liquids as a powerful class of catanionic hydrotropes. Green Chem. 2015, 17, 3948–3963.
- Shamshina, J.L.; Barber, P.S.; Rogers, R.D. Ionic liquids in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 1367–1381.
- Castro, L.; Pereira, P.; Freire, M.; Pedro, A. Progress in the Development of Aqueous Two-Phase Systems Comprising Ionic Liquids for the Downstream Processing of Protein- Based Biopharmaceuticals. Am. Pharm. Rev. 2019, 1–6.
- Ventura, P.M.; Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052.
- McQueen, L.; Lai, D. Ionic liquid aqueous two-phase systems from a pharmaceutical perspective. Front. Chem. 2019, 7.
- Egorova, K.S.; Ananikov, V.P. Biological Activity of Ionic Liquids Involving Ionic and Covalent Binding: Tunable Drug Development Platform. In Encyclopedia of Ionic Liquids; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–8. ISBN 9789811067396.
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189.
- Tanner, E.E.L.; Curreri, A.M.; Balkaran, J.P.R.; Selig-wober, N.C.; Yang, A.B.; Kendig, C.; Fluhr, M.P.; Kim, N.; Mitragotri, S. Design Principles of Ionic Liquids for Transdermal Drug Delivery. Adv. Mater. 2019, 31, 1901103.
- Dobler, D.; Schmidts, T.; Klingenhöfer, I.; Runkel, F. Ionic liquids as ingredients in topical drug delivery systems. Int. J. Pharm. 2013, 441, 620–627.
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. Med. Chem. Commun. 2016, 7, 1881–1897.
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic Liquids in Pharmaceutical Applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546.
- Huang, W.; Wu, X.; Qi, J.; Zhu, Q.; Wu, W.; Lu, Y.; Chen, Z. Ionic liquids: Green and tailor-made solvents in drug delivery. Drug Discov. Today 2020, 25, 901–908.
- Frizzo, C.P.; Gindri, I.M.; Tier, A.Z.; Buriol, L.; Moreira, D.N.; Martins, M.A.P. Pharmaceutical Salts: Solids to Liquids by Using Ionic Liquid Design. In Ionic Liquids—New Aspects for the Future; IntechOpen: London, UK, 2013; pp. 557–579.
- Moniruzzaman, M.; Mahmood, H.; Goto, M. Chapter 15: Ionic Liquid Based Nanocarriers for Topical and Transdermal Drug Delivery. In Ionic Liquid Devices; Royal Society of Chemistry: London, UK, 2017; pp. 390–403.