Bisphenol A (BPA) is a well-known endocrine disruptor present in epoxy resins and polycarbonate plastics, which negatively disturbs the male reproductive system affecting male fertility. In vivo studies showed that BPA exposure has deleterious effects on spermatogenesis by disturbing the hypothalamic-pituitary-gonadal axis and inducing oxidative stress in the testis. This compound seems to disrupt hormone signalling even at low concentrations, modifying the levels of inhibin B, oestradiol, and testosterone. The adverse effects on seminal parameters are mainly supported by studies based on urinary BPA concentration, showing a negative association between BPA levels and sperm concentration, motility, normal morphology and sperm DNA damage.
- Bisphenol A
- endocrine disruptors
- male infertility
- oxidative stress
- antioxidants
- phytochemicals
- medicinal plants
1. Introduction
Environment and diet strongly influence spermatogenesis, having significant consequences on male fertility and reproductive potential. There was rising concern about human exposure to endocrine-disrupting chemicals (EDCs) and their release into the environment [1,2][1][2]. An endocrine disruptor is defined by the World Health Organization as “an exogenous substance or mixture that alters the function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub)populations” [3]. EDCs may act by mimicking the biological activity of a hormone (agonistic effect), blocking its activity by binding to the receptor without activating it (antagonistic effect) or interfering with the synthesis or elimination rates of the natural hormones, even at extremely low doses (picomolar to nanomolar) [4,5][4][5]. Indeed, an important feature of EDCs is their unusual dose-response dynamics (usually inverted-U or U-shaped curves), since low doses may in some cases exert more potent effects than higher doses [5,6][5][6]. This characteristic, called non-monotonic response, complicates the assessment of potential impacts of exposure and makes the use of a dose test to predict low-dose effects inappropriate [5]. Moreover, it is important to consider that environmental exposure usually involves EDC mixtures, whose constituents can act through a common mode or by several mechanisms of action which might crosstalk [4,6][4][6]. This combined effect may have an additive, synergistic or attenuative potential [4,6][4][6]. EDC exposure during foetal development infancy, childhood and puberty can have long-lasting health effects since, at these moments, hormones strongly regulate the formation and maturation of organs. Early-life exposures have also been associated with developmental abnormalities and may increase the risk of several diseases later-in-life [4]. In adulthood, increasing incidences of several human reproductive disorders, such as testicular cancers and reduced sperm counts, may be partially attributed to increased exposure to environmental EDCs that have estrogenic activity [7,8,9,10,11][7][8][9][10][11].
Bisphenol A (BPA) represents one of those environmental chemical pollutants that mimic the natural oestrogen 17-β-oestradiol (E2). Epidemiological data from the US revealed that 90% of the general population have detectable levels of BPA in urine [12,13][12][13]. Its widespread presence in several daily used products and its detection in several human tissues and body fluids (urine, blood, serum, amniotic fluid, and semen) [14,15,16][14][15][16] raised many concerns about its potential association with human disorders such as cancer, cardiovascular diseases, obesity, diabetes, and reproductive disorders [7,13,17][7][13][17]. Although BPA may be toxic for other organs, attention has been paid to its reproductive and endocrine-disrupting effects [18,19,20][18][19][20]. The toxicity of BPA, especially at the reproductive level, results from its interaction with androgen and oestrogen receptors [21,22,23,24][21][22][23][24]. Although BPA is not an oxidizer itself, it leads to cellular changes usually manifested by lipid peroxidation (LPO) and free radicals production causing oxidative stress (OS) [21,22,23,24][21][22][23][24]. In the past decade, the use of antioxidants such as melatonin [25[25][26],26], vitamin C [27], N-acetylcysteine [28], coenzyme Q10 [29], and several plant extracts [30,31[30][31][32],32], to prevent and/or revert BPA-induced testicular toxicity started to be investigated.
2. BPA: What Is This?
Bisphenol A (4,40-isopropylidenodi-phenol compound 2,2-bis (4-hydroxylphenyl)-propane) is a crystalline chemical compound widely used as a monomer in the industry to produce plastic materials (polycarbonate, phenol, and epoxy resins), polyesters, and polyacrylate. During the past 50 years, this compound has often been used as an additive and/or antioxidant in polyvinyl chloride (PVC) production and processing, cosmetics and as a plastic softener [33]. Among many applications, this compound is present in several daily use products, such as containers to line food and beverage, plastic dishes, kitchen utensils, dental sealants and fillers, electronics (fridges, hairdryers, cell phones, computers) and thermal paper [34]. Due to its resiliency, flexibility, and durability, BPA has also been used in the manufacture of arms, safety equipment (helmets), and medical devices [34]. As a component of epoxy resins, BPA is also present on the internal coating of cans used in canned food [35]. The main route of exposure of BPA is dietary ingestion since the exposure to temperatures higher than 70 °C and the reutilization of containers results in BPA leakage to food and beverage [36,37][36][37]. However, the risk of exposure through inhalation [38,39,40][38][39][40] and skin contact, especially through thermal paper [41,42[41][42][43],43], is also considerable.
After entering the organism, arround 12% of BPA is metabolized in the liver by glucuronidation—BPA quickly binds to glucuronic acid by the liver enzyme uridine diphosphonate glucuronosyltransferase (UGT) producing BPA glucuronide (BPA-G) [16,44][16][44]. This process increases BPA water solubility with a consequent faster excretion in urine (half-life of elimination of 5.4–6.4 h), which means that humans exposed to oral doses of BPA ranging from 50 to 100 µg/kg body weight have less than 1% free BPA after 24 h [44]. The possible BPA bioaccumulation in the liver associated with its ingestion remains a topic of debate. However, contrary to dietary exposure, almost all BPA resulting from transdermal exposure avoids the liver metabolism, resulting in significantly higher concentrations of the unconjugated form (free BPA) in the bloodstream [45,46][45][46]. Considering that only free BPA has a biologically active role, the effects of transdermal exposure on human’s health represents a major concern. Currently, total urinary BPA (conjugated and unconjugated forms) is generally used as a biomarker of exposure to this chemical [47].
Considered an EDC, BPA disturbs the normal hormonal signalling resulting in adverse effects for the whole organism. In 1998, Gould [48] and Kuiper [49] showed that free BPA interacts with oestrogen receptor α (ERα), activating it in a manner distinct from the classical pattern observed in weak oestrogens, partial agonists and antagonists. Recently, it was reported that free BPA binds several nuclear receptors by (i) mimicking the action of endogenous steroids, (ii) maintaining the target molecule in active conformations and (iii) blocking the access of endogenous E2 to the receptor’s binding site by competition [18,50][18][50]. However, based on the available evidence, BPA has a very weak binding affinity to the oestrogen receptor, being almost 10,000 times weaker than that of natural E2 [51]. Additionally, it may also bind to other receptors such as G protein-coupled oestrogen receptor 30 (GPR30/GPER1) [52[52][53],53], orphan nuclear oestrogen-related receptor gamma (ERR-γ) [54[54][55],55], androgen receptor (AR), peroxisome proliferator-activated receptor gamma (PPAR-γ), and thyroid hormone receptor (TR) [56]. The binding to these receptors may lead to other alterations in cells and tissues rather than endocrine disturbance.
Considering that several studies showed deleterious effects of BPA exposure, several BPA Product Regulations have been created. Regulation (EU) 10/2011 and its amendment Regulation (EU) 2018/213 banned in European Union the use of BPA in feeding bottles, plastic cups and packaging containing food intended to be used by infants and children younger than 3 years old; and introduced stricter limits on BPA in food contact materials [57,58][57][58]. Since 2020, REACH directives (Regulation (EC) No 1907/2006) mandates that thermal paper cannot contain a BPA concentration equal to or greater than 0.02% by weight. Moreover, several European countries adopted their own measures regarding BPA. For instance, Sweden (Regulation SFS 2012:991), Belgium (Act of 4 September 2012), and Denmark (Statutory Order No. 822) prohibited BPA in food contact materials for infants and children under the age of 3 years old; France (Law No. 2012-1442) forbidden BPA in all food packaging intended to be in direct contact with food. However, the recent results of the CLARITY (Consortium Linking Academic and Regulatory Insights on BPA Toxicity)-BPA study intensified the controversy around this topic. This study was conducted by a consortium of US government scientists and several academic research groups having two components—the core study [59] and 14 grantee studies [60]. The core study consisted in three groups of pregnant rats (control group, BPA-exposed and oestrogen exposed), in which the female rats and the offspring were exposed to different concentrations of BPA throughout their whole lifespan (continuous dose), or by “stop-dose“ [59,61,62][59][61][62]. Several tissues were examined (brain, heart, mammary gland, ovaries, prostate, testis, etc.) to determine if (a) the continuous exposure was directly relevant for human exposure and safety assessment, (b) the “stop-dose” exposure can be effectively used to investigate whether developmental exposure shows adverse effects later in life, and (c) the effects at low doses and/or non-monotonic dose–responses could be seen [61,63][61][63]. Overall, the results indicated that there was no evidence of non-monotonic dose-response or relevant adverse effects of developmental exposure later in life [61]. The authors concluded that BPA is safe for consumers at typical consumer exposure levels. As the European Food Safety Authority (EFSA) started a re-evaluation of the safety of BPA for food contact applications in 2017 that will include the CLARITY-BPA study, it is possible that some policies may be updated.
3. BPA-Induced Alterations in Testicular Structure, Function, and Semen Parameters
In the past century, increasing attention has been paid to BPA effects on human’s health [64,65][64][65]. Since then, the associations between BPA levels and testicular toxicity, semen parameters, and overall male fertility have been extensively studied. Importantly, the severity of BPA impact on the male reproductive system depends on age, dose, mode, and duration of exposure [19,66][19][66]. In fact, methodological differences and distinct study populations can explain some of the contradictory results. In in vivo studies, BPA is typically administered in rodents orally. The doses usually range from 0.05–1 mg/kg/day for 30 days to 10 mg/kg/day during two weeks in mice. Rats were generally exposed to higher concentrations of BPA, ranging from 25 mg/kg/day during 60 days to 200 mg/kg/day for 10–30 days. Moreover, BPA and its metabolites have been measured in the plasma (<LLOQ (0.0435 µg/L)–7.23 µg/L; median 0.093 µg/L [67]), blood (0.19 ± 0.16 µg/L [68]), urine (1.66 ± 1.31 µg/L [68]), and seminal fluid (<LLOQ (0.0289 µg/L)–10.9 µg/L; median 0.085 µg/L [67]) in men. Based on new toxicological data and methodologies, the European Authorities adjusted the tolerable daily intake from 50 to 4 µg/kg/day, which may be revised soon according to the results of the CLARITY-BPA study [59].
The most significant risks associated with BPA exposure are attributed to its action as an EDC. It was shown that BPA has estrogenic activity deregulating the hypothalamic-pituitary-gonadal (HPG) axis even at low concentrations (Figure 1). Studies performed in animal models showed that BPA directly acts on Leydig cells, reducing their proliferation [32] and impairing the normal steroidogenesis by promoting (i) the production of 17- hydroxy-pregnenolone and testosterone from cholesterol, (ii) the expression of CYP19A1 that converts testosterone into E2, resulting in higher levels of the latter [69], and by reducing the expression of the steroidogenic enzyme 17α-hydroxylase/17–20 lyase [70]. Consistent with this finding, several in vitro and in vivo studies reported that BPA negatively affects testosterone production in both mice [71,72][71][72] and rat models [24,70,73[24][70][73][74],74], as well as in humans [14,73][14][73]. Moreover, BPA indirectly suppresses the synthesis and release of luteinizing hormone (LH) from the pituitary [70,71][70][71] through aromatase upregulation in testes, activating the mechanisms of negative hormonal feedback [71]. Additionally, human epidemiological studies showed that BPA modulates the levels of follicle-stimulating hormone (FSH) [75[75][76],76], inhibin B [76[76][77],77], and E2 [76,77][76][77] in men. Interestingly, prenatal exposure to BPA resulted in abnormal foetal development and testicular endocrine function, associated with reduced Leydig cell proliferation and foetal testosterone production [72,73,74][72][73][74]. All these alterations result in impaired testosterone production, with consequent effects on spermatogenesis [78,79][78][79] (Figure 1).
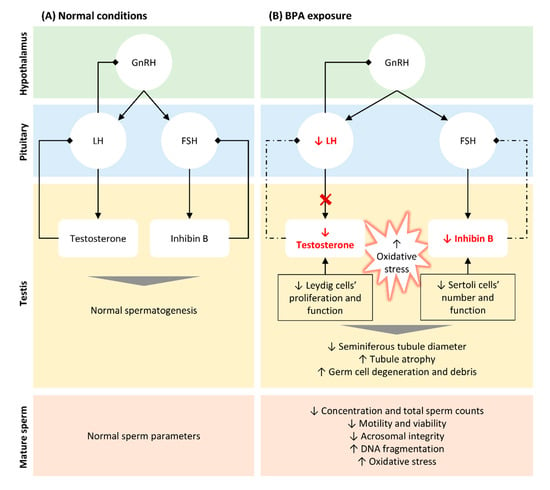
Figure 1. Schematic representation of BPA-induced alterations in hypothalamic-pituitary-testicular (HPT) axis, testicular function and structure, and in seminal parameters. (A). In normal conditions, gonadotrophin-releasing hormone (GnRH) is released by the hypothalamus stimulating the secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) by the pituitary. LH acts on Leydig cells and FSH on Sertoli cells, stimulating the biosynthesis of testosterone and inhibin B, respectively. Both hormones are crucial for normal spermatogenesis and, thus, to produce normal sperm. When testosterone and inhibin are released in the bloodstream, they inhibit GnRH/LH and FSH secretion, respectively (negative feedback). (B). Even at low concentrations, BPA reduced the levels of testosterone by directly targeting Leydig cells, reducing their proliferation, and impairing normal steroidogenesis. Moreover, BPA indirectly suppresses the release of LH through aromatase upregulation in testis, blocking testosterone synthesis. The reduction of inhibin B observed following BPA exposure is also associated with a reduction in the number of Sertoli cells, directly affecting spermatogenesis. The lower levels of testosterone and inhibin B block the mechanism of negative feedback, in an attempt to increase the release of LH and FSH and their action in testis (dashed arrows). BPA exposure also results in an increase in free radicals, which associated with altered hormonal levels lead to histological alterations in testis and germ cells’ reduction and degenerations. These alterations explain the abnormal seminal parameters observed in situations of BPA exposure, such as decreased concentration and total sperm count, reduced motility and viability and increased DNA fragmentation.
4. Impact of BPA Exposure on Oxidative Stress in Testis and Sperm
The imbalance between the excessive production of reactive oxygen species (ROS) and their neutralization and removal by the antioxidant system results in an increase in OS [109][80]. Cells present a complex system of antioxidant defences that contains antioxidant enzymes, molecular antioxidants, and metallic chemical agents, converting ROS into non-toxic forms [51]. Enzymatic antioxidants include superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT), which protect the living system from the harmful effects of ROS and reduce their oxidative damage to cell membranes [51,110][51][81] (Figure 2). SOD constitutes the first line of defence against superoxide radicals (O2−) by catalysing their dismutation to form hydrogen peroxide (H2O2) and oxygen (O2) [111][82]. H2O2 causes rapid and severe oxidative damage to lipids, proteins, and DNA [110][81]. Reduced SOD activity results in the accumulation of O2−, which in turn inhibits CAT activity, decreasing the cells ability to eliminate H2O2 [51]. On the other hand, GPx may act directly as an antioxidant enzyme, catalysing the reduction of phospholipid hydroperoxides within membranes and lipoproteins [112][83]. Since high ROS levels promote the oxidation of biomolecules such as nucleic acids, lipids, and proteins, these enzymes constitute important intracellular antioxidants to protect against ROS-mediated damage [111,113][82][84]. Molecular antioxidants are typically scavenging/non-enzymatic antioxidants (NADPH, glutathione, vitamins, flavonoids, carotenoids, melatonin) that bind to active free radicals and disrupt chain propagation reactions [51]. These antioxidants donate an electron to free radicals to neutralize them, becoming free radicals with reduced toxicity that are easily neutralized by other antioxidants in the same class. Finally, some metals, such as zinc (Zn) have important antioxidant and anti-inflammatory properties [114,115][85][86]. Zinc, copper (Cu), and iron (Fe) are essential components of the antioxidant enzymes Cu-SOD, Zn-SOD, and CAT, respectively [116][87]. Indeed, it was reported that Zn deficiency aggravates the toxicity of BPA in rat testis, increasing cellular and DNA damage, apoptosis, and modifying protein expression [117][88].
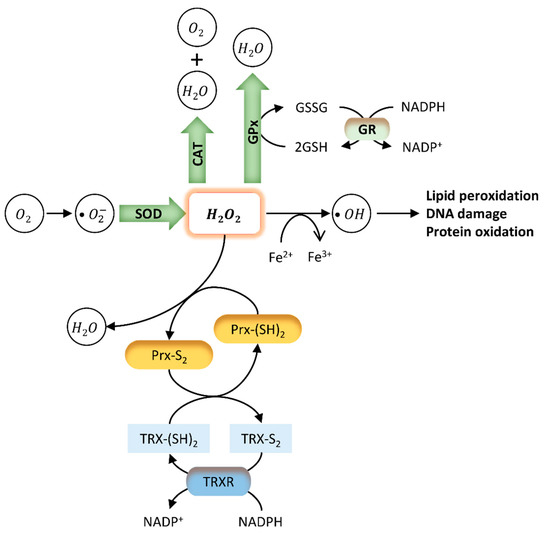
Figure 2. Endogenous antioxidant mechanisms. The oxidative stress (OS) caused by several external and internal factors leads to excessive production of ROS intracellularly, including the free radicals hydroxyl (OH−), peroxyl (HO2), and superoxide (O2−). Cellular redox homeostasis is maintained by an endogenous antioxidant defence system that includes the endogenous antioxidant enzymes SOD, CAT, GPx, and GSH (green). These antioxidants directly scavenge O2− and hydrogen peroxide (H2O2), converting them into less reactive species. SOD catalyses the dismutation of superoxide radicals to H2O2. H2O2 is rapidly converted into OH- radical, which is very reactive and causes lipid peroxidation and DNA damage. GPx neutralizes H2O2 by taking hydrogens from two GSH molecules resulting in two H2O and one GSSG. GR then regenerates GSH from GSSG. Finally, CAT neutralizes H2O2 into H2O and O2. Two other antioxidant systems involve peroxiredoxins (Prx) (yellow) and the thioredoxin (TRX) system (blue). Prx are ubiquitous antioxidant enzymes that catalyse the reduction of H2O2, peroxynitrite (ONOO−), and organic hydroperoxides to water, nitrite, or hydroxyl derivatives (ROH), respectively [118][89]. The TRX system composed by TRX, TRX reductase (TRXR), and NADPH is a ubiquitous thiol oxidoreductase system that also regulates cellular redox status [119][90]. Briefly, initial oxidation of the active site of Prx forms an interchain disulphide (Prx-S2). The hyper-oxidation of Prx decreases localized peroxidase activity, leading to the oxidation of less sensitive proteins [118][89]. Reduced TRX (TRX-(SH)2) catalyses the reduction of disulphides (S-S) within oxidized proteins, including Prx - Prx-(SH)2. In this process, Trx becomes oxidized (TRX-S2), being further reduced by thioredoxin reductase (TRXR) at the expense of NADPH.
5. Ameliorative Effects of Antioxidants in BPA-Induced Reproductive Toxicity
In the past years, several research groups have focused their investigation on possible approaches to treat or prevent BPA-induced testicular toxicity and male infertility. Since the effect of BPA on testicular cells and mature spermatozoa are particularly due to OS, most of the pharmacological approaches are based on the use of compounds with antioxidant properties (Table 1). Antioxidants are reducing agents capable of scavenging and neutralize free radicals, inhibiting oxidation and preventing OS in cells and tissues.
Table 1. Antioxidants used to treat or prevent BPA-induced male fertility and their effects. The animal model used in each study, the experimental design, and the effects of the coadministration of BPA and antioxidant compared with the effects of BPA exposure alone were also presented.
Reference | Animal | Antioxidant | Experimental Groups (G) | Effects of BPA + Antioxidant Administration | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
[21] |
Swiss albino mice | (in vivo) | Melatonin | (hormone) | G1: 0.2 mL olive oil (control); G2: 10 mg/kg BPA suspended in olive oil; G3: 10 mg/kg melatonin; G4: BPA 10 mg/kg + 10 mg/kg of melatonin—dose/day for 14 days | ↑ Mitochondrial marker enzymes SDH, MDH, IDH, NDH, MAO, GSH, antioxidant enzymes GPx, SOD, GR | ↓ LPO | ||||||||||||||||
|
[25] |
Sprague Dawley rats | (in vivo) | Melatonin | (hormone) | G1: 0.5% ethanol in normal saline (control); G2: 200 mg/kg BPA suspended in olive oil; G3: 10 mg/kg melatonin intraperitoneally 30 min before BPA administration; G4: 10 mg/kg melatonin intraperitoneally + 200 mg/kg BPA suspended in olive oil—dose/day for 10 days | ↔ body weight, reproductive organs weight, testes/body and epididymis/body weight ratios, sperm counts and apoptosis | ↑ SOD activity and 4C-cells number | ↓ TBARS accumulation and DNA damage in spermatocytes, number of γH2AX-positive foci | |||||||||||||||
|
[26] |
Sprague Dawley rats | (in vivo) | Melatonin | (hormone) | G1: no treatment (normal control); G2: 0.2 mL corn oil (experimental control); G3: normal saline (experimental control); G4: 50 mg/kg BPA suspended in corn oil; G5: 10 mg/kg melatonin in normal saline; G6: 10 mg/kg melatonin + 50 mg/kg BPA—3 days/week for 6 weeks | ↑ sperm count and motility, testosterone levels, GSH, viable cells | ↓ mortality and abnormal sperm, % diploid sperm and spermatid; levels of H 2O2 and MDA, necrotic and apoptotic cells | Other alterations: seminiferous tubules showed increase in the germinal cell population with active spermatogenesis and normal arrangement of spermatogenic cell, Leydig cells population normal | |||||||||||||||
|
[85] |
[91] |
Sprague Dawley rats | (in vivo) | Melatonin | (hormone) | G1: 25 mg/kg sesame oil + 25 mg/kg 0.1% ethanol (control); G2: 25 mg/kg BPA; G3: 25 mg/kg BPA + 20 mg/kg melatonin—dose/day for 60 days | ↔ total sperm counts | ↑ Cldn-1, Occ and ZO-1 immunostaining, sperm motility | Other alterations: Fewer vacuolations, irregular tubules and degenerative cells containing a heterochromatic nucleus in epididymis | ||||||||||||||
|
[86] |
[92] |
Wistar albino rats | (in vivo) | Melatonin | (hormone) | G1: 0.2 mL 1% dimethyl sulfoxide (DMSO)/99% canola oil (control); G2: 0.025 mg/kg BPA; G3: 0.25 mg/kg BPA; G4: 0.025 mg/kg BPA + melatonin 1 mg/kg; G5: 0.25 mg/kg BPA + melatonin 1 mg/kg—dose/day; exposure in utero from gestational day 10–21 | ↑ body weight; gonosomatic index; sperm motility; viability and count; serum T levels and LH; activity of SOD, GSH, GPx, and GST; tubular and luminal diameter | ↓ FSH and E2; testicular MDA and H 2O2 levels, interstitial necrosis, and germinal cell degeneration | |||||||||||||||
|
[134] |
[93] |
Wistar albino rats | (in vivo) | Folic acid | (vitamin B9) | G1: 0.5 mL 0.9% NaCl (control); G2: 50 mg/kg BPA in 0.5 mL corn oil; G3: 20 mg/kg/day folic acid in 0.5 mL 0.9% NaCl; G4: 20 mg/kg folic acid in 0.5 mL 0.9% NaCl + 50 mg/kg BPA in 0.5 mL corn oil—dose/day for 14 days | ↔ body weight, testes/body weight ratios, number of UTF-1 positive cells/tubule and UTF-1 positive tubules | ↑ serum testosterone levels, viable sperm | ↓ TUNEL positive cells and tubules, head, midpiece and total sperm abnormalities | ||||||||||||||
|
[27] |
Wistar albino rats | (in vivo) | Vitamin C | G1: olive oil (control); G2: 25 mg/kg/day BPA; G3: 25 mg/kg/day BPA + 60 mg/kg/day of vitamin C three times a week—50 days | ↑ right epididymal weight, congestion areas, atrophy, germinal cell debris | ↓ GSH | |||||||||||||||||
|
[129] |
[94] |
CD-1 (ICR) mice | (in vitro) | Vitamin C, Vitamin E and GSH | Condition I: DMSO (control); Cond II: 100 µM BPA; Cond III: 100 µM BPA + 5 mM GSH; Cond IV: 100 µM BPA + 100 µM Vitamin C; Cond III: 100 µM BPA + 2 mM of Vitamin E—for 6 h | ↑ sperm motility, ATP levels | ↓ acrosome-reacted spermatozoa, PKA activity, protein tyrosine phosphorylation and nitration, ROS levels | ||||||||||||||||
|
[135] |
[95] |
SHN mice | (in vivo) | Vitamin A | G1: 16 mL of sesame oil and 4 mL of dimethyl sulfoxide (control); G2: 0.5 mg BPA; G3: 50 mg BPA; G4: 50 mg BPA + 100 IU Retinoic Acid—for 5 days from the date of birth | ↑ sperm motility | ↓ abnormal sperm | ||||||||||||||||
|
[31] |
Human | (in vitro) | Eruca Sativa aqueous extract | Condition I: untreated (control); Cond II: 10 µM BPA; Cond III: 10 µM BPA + 15.5 µg/mL ESAE; Cond IV: 10 µM BPA + 62.55 µg/mL ESAE; Cond V: 10 µM BPA + 250 µg/mL ESAE; Cond VI: 10 µM BPA + 1000 µg/mL ESAE—ESAE incubation for 1 h followed by BPA incubation for 4 h | ↑ sperm progressive motility and viability, mitochondrial function | ↓ immotile sperm | |||||||||||||||||
|
[32] |
Wistar albino rats | (in vivo) | Eruca Sativa aqueous extract | G1: 0.4 mL/kg/day of tocopherol-stripped corn oil (control); G2: 100 mg/kg BPA; G3: 200 mg/kg ESAE; G4: 100 mg/kg BPA + 50 mg/kg ESAE; G5: 100 mg/kg BPA + 100 mg/kg ESAE; G6: 100 mg/kg BPA + 200 mg/kg ESAE—dose/day for 30 days | ↑ body weight, reproductive organs weight, testosterone, and LH levels, sperm counts, motility, viability, SH group content | ↓ morphologically abnormal sperm; MDA levels; SOD, CAT and GPx activities | |||||||||||||||||
|
[30] |
Sprague Dawley rats | (in vivo) | Cordyceps militaris | G1: no intervention (normal control); G2: 200 mg/kg BPA; G3: 800 mg/kg C.militaris; G4: 200 mg/kg BPA + 200 mg/kg C. militaris; G5: 200 mg/kg BPA + 400 mg/kg C. militaris; G6: 200 mg/kg BPA + 800 mg/kg C. militaris; G7: 200 mg/kg BPA + 300 mg/kg Vitamin E—28 days | ↑ body weight; SOD, GPx, GSH, testosterone, and LH serum levels; sperm counts and motility; mRNA levels of Star; CYP11A1; 3β-HSD; and CYP17A1 | ↓ MDA levels | |||||||||||||||||
|
[87] |
[96] |
Sprague Dawley rats | (in vivo) | Cistanche tubulosa and Echinacoside (ECH) | G1: corn oil 10 mL/kg (normal control); G2: 200 mg/kg BPA; G3: 200 mg/kg BPA + 300 mg/kg Vitamin E; G4: 200 mg/kg BPA + 6 mg/kg ECH; G5: 200 mg/kg BPA + 200 mg/kg CT; G6: 6 mg/kg EC; G7: 200 mg/kg CT—6 weeks | ↑ sperm motility; LDH-x activity; FSH, LH, and testosterone serum levels; mRNA levels of StAR, CYP17A1, 3β-HSD, and 17β-HSD; protein levels of CYP11A1 and CYP17A1 | ↓ abnormal sperm | Other alterations: normal histological pattern, normal spermatogenic series | |||||||||||||||
|
[88] |
[97] |
Wistar albino rats | (in vivo) | Naringin | (flavonoid) | G1: Control; G2: 50 mg/kg BPA; G3: 50 mg/kg BPA + 40 mg/kg naringin; G4: 50 mg/kg BPA + 80 mg/kg naringin; G5: 50 mg/kg BPA + 160 mg/kg naringin; G6: 160 mg/kg Naringin—for 30 days | ↔ body weight | ↑ testicular weight and volume; total testicular protein; epididymal sperm count; testicular enzymes (ALP, LDH); serum FSH; LH; testosterone and E2; activities of GPx, SOD, and CAT; GSH | ↓ MDA, ROS | Other: less testicular tissue damage | |||||||||||||
|
[89] |
[98] |
Sprague Dawley rats | (in vivo) | Quercetin | (flavonoid) | G1: normal saline (control); G2: 50 mg/kg BPA; G3: 50 mg/kg quercetin; G4: 50 mg/kg BPA + 50 mg/kg quercetin—for 52 days | ↔ body weight, reproductive organ weight | ↑ plasma testosterone, LDL and HDL levels, tunica albuginea thickness, seminiferous tubule area, number of spermatogonia, primary spermatocytes, secondary spermatocytes, and spermatids | ↓ oestrogen levels, blood urea nitrogen levels, creatinine, cholesterol, triglyceride levels | ||||||||||||||
|
[90] |
[99] |
Balb/c mice | (in vivo) | Trigonella foenum-graecum | G1: normal pellet diet (control); G2: 200 mg/kg fenugreek seeds aqueous extract; G3: 1 mg/kg BPA; G4: 1 mg/kg BPA + 200 mg/kg fenugreek seeds aqueous extract—2 months | ↑ testis weight, sperm concentration, sperm motility, GSH, GPx activity, Bcl-2 mRNA levels | ↓ ROS and LPO, Caspase-9 and -3 mRNA level | Other alterations: improved histoarchitecture, basement membrane preservation with less vacuolization and increased number of elongated, round spermatids | |||||||||||||||
|
[131] |
[100] |
CD-1 (ICR) mice | (in vivo) | Lespedeza cuneata ethanol extract (LCE) | G1: normal saline (solvent control); G2:10 mg/kg BPA; G3: 10 mg/kg BPA + 100mg/kg Saw Palmetto extract (SPE); G4: 10 mg/kg BPA + 25 mg/kg LCE; G5: 10 mg/kg BPA + 50 mg/kg LCE; G6: 10 mg/kg BPA + 100 mg/kg LCE—for 12 weeks | ↑ testis weight; sperm counts and motility; testosterone levels; GSH, CAT, and SOD1 levels; HDL-cholesterol | ↓ sperm abnormalities; TBARS levels; glucose; TC, TG, and LDL- cholesterol | ||||||||||||||||
|
[132] |
[101] |
Sprague Dawley rats | (in vivo) | Lycopene | (carotenoid) | G1: saline following treatment with 0.5 mL corn oi (control); G2: 200 mg/kg BPA; G3: 200 mg/kg BPA + 10 mg/kg lycopene; G4: 10 mg/kg lycopene—for 30 days | ↑ body and organ weight, sperm count, sperm motility, antioxidants enzymes level (SOD, CAT, GPx, GR) | ↓ LPO and H 2O2 | |||||||||||||||
|
[130] |
[102] |
NMRI mice | (in vitro) | Taurine | (amino acid) | Condition I: untreated (control); Cond II: 0.8 mmol/L BPA for 2 h; Cond III: 50 µmol/ L TAU for 4 h; Cond IV: pre-treated with 5 µmol/L of TAU for 2 h before BPA treatment (2 h); Cond V: pre-treated with 10 µmol/L of TAU for 2 h before BPA treatment (2 h); Cond VI: pre-treated with 30 µmol/L of TAU for 2 h before BPA treatment (2 h); Cond VII: pre-treated with 50 µmol/L of TAU for 2 h before BPA treatment (2 h) | ↑ Sperm and testicular mitochondria viability, MMP, GSH, SOD, sperm motility | ↓ testicular mitochondrial ROS, MDA | |||||||||||||||
|
[22] |
BALB/c mice | (in vivo) | Selenium | G1: diet adequate in selenium (0.2 ppm/kg diet) as sodium selenite for 12 weeks (control); G2: 0.5 ppm sodium selenite/kg for 12 weeks; G3: 0.2 ppm sodium selenite/kg for 8 weeks followed by 1 mg/kg BPA for 4 weeks; G4: 0.5 ppm sodium selenite/kg for 8 weeks followed by 1 mg/kg BPA for 4 weeks | ↑ sperm concentration and motility, GPx activity | ↓ ROS and LPO levels, number of TUNEL- positive germ cells | Other alterations: preserved basement membrane with less vacuolization, increased germ cell count | ||||||||||||||||
|
[28] |
Gobiocypris rarus | (in vivo) | NAC | G1: 0.001%DMSO (control); G2: 10 mg/kg NAC; G3: 100 mg/kg NAC; G4: 225 μg/L BPA; G5: 10 mg/kg NAC + 225 μg/L BPA; G6: 100 mg/kg NAC + 225 μg/L BPA — for 7 days | ↑ GPx activity | ↓ levels of 5-methylcytosine (5mC), GSH, γ-glutamyl cysteine synthetase (GCS), DNA methyltransferase proteins (DNMTs), H 2O2 concentration, S-adenosylhomocysteine (SAH), homocysteine (HCY), nicotinamide adenine dinucleotide phosphate (NADPH) levels, SOD, CAT activities | |||||||||||||||||
|
[101] |
[103] |
Wistar albino rats | (in vivo) | NAC | 0, 1.0 or 10 mg/L BPA for 8 weeks and BPA + 0.45% NAC for 2 days prior to the administration of BPA | ↑ sperm motility | ↓ HNE-modified protein at 30 kDa, ROS levels |
Legend: ↔ no change; ↑ increase; ↓ decrease.
6. Conclusions
BPA is now recognized as a potent endocrine disruptor that compromises the HPG axis during foetal and adult life and disturbs the cellular redox balance in testis and sperm, resulting in altered testis development, architecture and function, impaired endocrine function, and abnormal semen parameters. Overall, available data support an adverse effect of BPA on sperm characteristics, such as reduced motility and concentration, and increased genetic abnormalities; however, these alterations were not accompanied by clear data on fertility outcomes. At the molecular level, increased ROS production, mitochondrial dysfunction, and redox imbalance seem to be important factors for BPA-induced testicular damage.
The recognition of effective markers of exposure able to determine and predict the health and reproductive consequences and the identification of therapeutic moieties capable of rescue the BPA-induced toxicity on the male reproductive system represent the major challenges in this field. Antioxidants that reduce OS, lipid peroxidation, and DNA damage, restoring the global antioxidant defence system, can be used to treat male infertility and poor semen quality associated with BPA exposure.
References
- Kasonga, T.K.; Coetzee, M.A.; Kamika, I.; Ngole-Jeme, V.M.; Momba, M.N.B. Endocrine-disruptive chemicals as contaminants of emerging concern in wastewater and surface water: A review. J. Environ. Manag. 2021, 277, 111485.
- Kelly, M.; Connolly, L.; Dean, M. Public Awareness and Risk Perceptions of Endocrine Disrupting Chemicals: A Qualitative Study. Int. J. Environ. Res. Public Health 2020, 17, 7778.
- European Commission. Community Strategy for Endocrine Disrupters—A Range of Substances Suspected of Interfering with the Hormone Systems of Humans and Wildlife; Commission of the European Communities: Brussels, Belgium, 1999.
- Fuhrman, V.F.; Tal, A.; Arnon, S. Why endocrine disrupting chemicals (EDCs) challenge traditional risk assessment and how to respond. J. Hazard. Mater. 2015, 286, 589–611.
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.J.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr. Rev. 2012, 33, 378–455.
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342.
- Balabanič, D.; Rupnik, M.; Klemenčič, A.K. Negative impact of endocrine-disrupting compounds on human reproductive health. Reprod. Fertil. Dev. 2011, 23, 403–416.
- Chiang, C.; Mahalingam, S.; Flaws, J.A. Environmental Contaminants Affecting Fertility and Somatic Health. Semin. Reprod. Med. 2017, 35, 241–249.
- Giwercmanz, A.; Rylander, L.; Giwercman, Y.L. Influence of endocrine disruptors on human male fertility. Reprod. Biomed. Online 2007, 15, 633–642.
- Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Skinner, M.K. Plastics Derived Endocrine Disruptors (BPA, DEHP and DBP) Induce Epigenetic Transgenerational Inheritance of Obesity, Reproductive Disease and Sperm Epimutations. PLoS ONE 2013, 8, e55387.
- Sharma, A.; Mollier, J.; Brocklesby, R.W.K.; Caves, C.; Jayasena, C.N.; Minhas, S. Endocrine-disrupting chemicals and male reproductive health. Reprod. Med. Biol. 2020, 19, 243–253.
- Calafat, A.M.; Ye, X.; Wong, L.-Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. Population to Bisphenol A and 4-tertiary-Octylphenol: 2003–2004. Environ. Health Perspect. 2008, 116, 39–44.
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of Urinary Bisphenol A Concentration with Medical Disorders and Laboratory Abnormalities in Adults. JAMA 2008, 300, 1303–1310.
- Vitku, J.; Sosvorova, L.; Chlupacova, T.; Hampl, R.; Hill, M.; Sobotka, V.; Heracek, J.; Bicikova, M.; Starka, L. Differences in Bisphenol A and Estrogen Levels in the Plasma and Seminal Plasma of Men with Different Degrees of Infertility. Physiol. Res. 2015, 64, S303–S311.
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177.
- Völkel, W.; Bittner, N.; Dekant, W. Quantitation of Bisphenol a and Bisphenol a Glucuronide in Biological Samples by High Performance Liquid Chromatography-Tandem Mass Spectrometry. Drug Metab. Dispos. 2005, 33, 1748–1757.
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155.
- De Toni, L.; Ponce, M.D.R.; Petre, G.C.; Rtibi, K.; Di Nisio, A.; Foresta, C. Bisphenols and Male Reproductive Health: From Toxicological Models to Therapeutic Hypotheses. Front. Endocrinol. 2020, 11, 1–9.
- Cariati, F.; D’Uonno, N.; Borrillo, F.; Iervolino, S.; Galdiero, G.; Tomaiuolo, R. Bisphenol A: An emerging threat to male fertility. Reprod. Biol. Endocrinol. 2019, 17, 1–8.
- Radwan, M.; Wielgomas, B.; Dziewirska, E.; Radwan, P.; Kałużny, P.; Klimowska, A.; Hanke, W.; Jurewicz, J. Urinary Bisphenol A Levels and Male Fertility. Am. J. Men Health 2018, 12, 2144–2151.
- Anjum, S.; Rahman, S.; Kaur, M.; Ahmad, F.; Rashid, H.; Ansari, R.A.; Raisuddin, S. Melatonin ameliorates bisphenol A-induced biochemical toxicity in testicular mitochondria of mouse. Food Chem. Toxicol. 2011, 49, 2849–2854.
- Kaur, S.; Saluja, M.; Bansal, M.P. Bisphenol A induced oxidative stress and apoptosis in mice testes: Modulation by selenium. Andrology 2018, 50, e12834.
- Hulak, M.; Gazo, I.; Shaliutina, A.; Linhartova, P. In vitro effects of bisphenol A on the quality parameters, oxidative stress, DNA integrity and adenosine triphosphate content in sterlet (Acipenser ruthenus) spermatozoa. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2013, 158, 64–71.
- D’Cruz, S.C.; Jubendradass, R.; Mathur, P.P. Bisphenol A Induces Oxidative Stress and Decreases Levels of Insulin Receptor Substrate 2 and Glucose Transporter 8 in Rat Testis. Reprod. Sci. 2011, 19, 163–172.
- Wu, H.-J.; Liu, C.; Duan, W.-X.; Xu, S.-C.; He, M.-D.; Chen, C.-H.; Wang, Y.; Zhou, Z.; Yu, Z.-P.; Zhang, L.; et al. Melatonin ameliorates bisphenol A-induced DNA damage in the germ cells of adult male rats. Mutat. Res. Toxicol. Environ. Mutagen. 2013, 752, 57–67.
- Othman, A.I.; Edrees, G.M.; El-Missiry, M.A.; Ali, D.A.; Aboel-Nour, M.; Dabdoub, B.R. Melatonin controlled apoptosis and protected the testes and sperm quality against bisphenol A-induced oxidative toxicity. Toxicol. Ind. Health 2016, 32, 1537–1549.
- Aydoğan, M.; Korkmaz, A.; Barlas, N.; Kolankaya, D. Pro-oxidant effect of vitamin C coadministration with bisphenol A, nonylphenol, and octylphenol on the reproductive tract of male rats. Drug Chem. Toxicol. 2009, 33, 193–203.
- Yuan, C.; Wang, L.; Zhu, L.; Ran, B.; Xue, X.; Wang, Z. N-acetylcysteine alleviated bisphenol A-induced testicular DNA hypermethylation of rare minnow (Gobiocypris rarus) by increasing cysteine contents. Ecotoxicol. Environ. Saf. 2019, 173, 243–250.
- Carneiro, M.F.H.; Shin, N.; Karthikraj, R.; Barbosa, F.; Kannan, K.; Colaiácovo, M.P. Antioxidant CoQ10 Restores Fertility by Rescuing Bisphenol A-Induced Oxidative DNA Damage in the Caenorhabditis elegans Germline. Genetics 2020, 214, 381–395.
- Wang, J.; Chen, C.; Jiang, Z.; Wang, M.; Jiang, H.; Zhang, X. Protective effect of Cordyceps militaris extract against bisphenol A induced reproductive damage. Syst. Biol. Reprod. Med. 2016, 62, 249–257.
- Grami, D.; Rtibi, K.; Selmi, S.; Jridi, M.; Sebai, H.; Marzouki, L.; Sabovic, I.; Foresta, C.; De Toni, L. Aqueous extract of Eruca Sativa protects human spermatozoa from mitochondrial failure due to bisphenol A exposure. Reprod. Toxicol. 2018, 82, 103–110.
- Grami, D.; Rtibi, K.; Hammami, I.; Selmi, S.; De Toni, L.; Foresta, C.; Marzouki, L.; Sebai, H. Protective Action of Eruca sativa Leaves Aqueous Extracts Against Bisphenol A-Caused In Vivo Testicular Damages. J. Med. Food 2020, 23, 600–610.
- Tomza-Marciniak, A.; Stępkowska, P.; Kuba, J.; Pilarczyk, B. Effect of bisphenol A on reproductive processes: A review of in vitro, in vivo and epidemiological studies. J. Appl. Toxicol. 2018, 38, 51–80.
- Fenichel, P.; Chevalier, N.; Brucker-Davis, F. Bisphenol A: An endocrine and metabolic disruptor. In Annales d’Endocrinologie; Elsevier: Amsterdam, The Netherlands, 2013; pp. 211–220.
- Almeida, S.; Raposo, A.; Almeida-González, M.; Carrascosa, C. Bisphenol A: Food Exposure and Impact on Human Health. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1503–1517.
- López-Cervantes, J.; Paseiro-Losada, P. Determination of bisphenol A in, and its migration from, PVC stretch film used for food packaging. Food Addit. Contam. 2003, 20, 596–606.
- Tan, B.; Mustafa, A. Leaching of Bisphenol A from New and Old Babies’ Bottles, and New Babies’ Feeding Teats. Asia Pac. J. Public Health 2003, 15, 118–123.
- Wilson, N.K.; Chuang, J.C.; Lyu, C.; Menton, R.; Morgan, M.K. Aggregate exposures of nine preschool children to persistent organic pollutants at day care and at home. J. Expo. Sci. Environ. Epidemiol. 2003, 13, 187–202.
- Wilson, N.K.; Chuang, J.C.; Morgan, M.K.; Lordo, R.A.; Sheldon, L.S. An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol-A, and nonylphenol at home and daycare. Environ. Res. 2007, 103, 9–20.
- Hines, C.J.; Christianson, A.L.; Jackson, M.V.; Ye, X.; Pretty, J.R.; Arnold, J.; Calafat, A.M. An Evaluation of the Relationship among Urine, Air, and Hand Measures of Exposure to Bisphenol A (BPA) in US Manufacturing Workers. Ann. Work. Expo. Health 2018, 62, 840–851.
- Porras, S.P.; Heinälä, M.; Santonen, T. Bisphenol A exposure via thermal paper receipts. Toxicol. Lett. 2014, 230, 413–420.
- Biedermann, S.; Tschudin, P.; Grob, K. Transfer of bisphenol A from thermal printer paper to the skin. Anal. Bioanal. Chem. 2010, 398, 571–576.
- Liao, C.; Kannan, K. Widespread Occurrence of Bisphenol A in Paper and Paper Products: Implications for Human Exposure. Environ. Sci. Technol. 2011, 45, 9372–9379.
- Thayer, K.A.; Doerge, D.R.; Hunt, D.; Schurman, S.H.; Twaddle, N.C.; Churchwell, M.I.; Garantziotis, S.; Kissling, G.E.; Easterling, M.R.; Bucher, J.R.; et al. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ. Int. 2015, 83, 107–115.
- Pottenger, L.H.; Domoradzki, J.Y.; Markham, D.A.; Hansen, S.C.; Cagen, S.Z.; Waechter, J.M. The Relative Bioavailability and Metabolism of Bisphenol A in Rats Is Dependent upon the Route of Administration. Toxicol. Sci. 2000, 54, 3–18.
- Tominaga, T.; Negishi, T.; Hirooka, H.; Miyachi, A.; Inoue, A.; Hayasaka, I.; Yoshikawa, Y. Toxicokinetics of bisphenol A in rats, monkeys and chimpanzees by the LC–MS/MS method. Toxicology 2006, 226, 208–217.
- Völkel, W.; Kiranoglu, M.; Fromme, H. Determination of free and total bisphenol A in human urine to assess daily uptake as a basis for a valid risk assessment. Toxicol. Lett. 2008, 179, 155–162.
- Gould, J.C.; Leonard, L.S.; Maness, S.C.; Wagner, B.L.; Conner, K.; Zacharewski, T.; Safe, S.; McDonnell, D.P.; Gaido, K.W. Bisphenol A interacts with the estrogen receptor α in a distinct manner from estradiol. Mol. Cell. Endocrinol. 1998, 142, 203–214.
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Van Der Saag, P.T.; Van Der Burg, B.; Gustafsson, J.-Å. Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor β. Endocrinology 1998, 139, 4252–4263.
- Li, L.; Wang, Q.; Zhang, Y.; Niu, Y.; Yao, X.; Liu, H. The Molecular Mechanism of Bisphenol A (BPA) as an Endocrine Disruptor by Interacting with Nuclear Receptors: Insights from Molecular Dynamics (MD) Simulations. PLoS ONE 2015, 10, e0120330.
- Amjad, S.; Rahman, S.; Pang, M.-G. Role of Antioxidants in Alleviating Bisphenol A Toxicity. Biomolecules 2020, 10, 1105.
- Dong, S.; Terasaka, S.; Kiyama, R. Bisphenol A induces a rapid activation of Erk1/2 through GPR30 in human breast cancer cells. Environ. Pollut. 2011, 159, 212–218.
- Wozniak, A.L.; Bulayeva, N.N.; Watson, C.S. Xenoestrogens at Picomolar to Nanomolar Concentrations Trigger Membrane Estrogen Receptor-α–Mediated Ca2+ Fluxes and Prolactin Release in GH3/B6 Pituitary Tumor Cells. Environ. Health Perspect. 2005, 113, 431–439.
- Matsushima, A.; Kakuta, Y.; Teramoto, T.; Koshiba, T.; Liu, X.; Okada, H.; Tokunaga, T.; Kawabata, S.; Kimura, M.; Shimohigashi, Y. Structural Evidence for Endocrine Disruptor Bisphenol A Binding to Human Nuclear Receptor ERR. J. Biochem. 2007, 142, 517–524.
- Okada, H.; Tokunaga, T.; Liu, X.; Takayanagi, S.; Matsushima, A.; Shimohigashi, Y. Direct Evidence Revealing Structural Elements Essential for the High Binding Ability of Bisphenol A to Human Estrogen-Related Receptor-γ. Environ. Health Perspect. 2008, 116, 32–38.
- Richter, C.A.; Birnbaum, L.S.; Farabollini, F.; Newbold, R.R.; Rubin, B.S.; Talsness, C.E.; Vandenbergh, J.G.; Walser-Kuntz, D.R.; Saal, F.S.V. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007, 24, 199–224.
- European Union. Commission Regulation (EU) No 10/2011 of 14 January 2011; Official Journal of the European Union: Brussels, Belgium, 2011; pp. 1–89.
- European Commission. Commission Regulation (EU) 2018/832 of 12 February 2018 on the Use of Bisphenol A in Varnishes and Coatings Intended to Come into Contact with Food and Amending Regulation (EU) No 10/2011 as Regards the Use of That Substance in Plastic Food Contact Materials; Official Journal of the European Union: Brussels, Belgium, 2018; p. 7.
- National Toxicology Program—NTP. NTP Research Report on the CLARITY-BPA Core Study: A Perinatal and Chronic Extended-Dose-Range Study of Bisphenol A in Rats; National Toxicology Program: Research Triangle Park, NC, USA, 2018.
- Schug, T.T.; Heindel, J.J.; Camacho, L.; Delclos, K.B.; Howard, P.; Johnson, A.F.; Aungst, J.; Keefe, D.; Newbold, R.; Walker, N.J.; et al. A new approach to synergize academic and guideline-compliant research: The CLARITY-BPA research program. Reprod. Toxicol. 2013, 40, 35–40.
- Camacho, L.; Lewis, S.; VanLandingham, M.; Olson, G.; Davis, K.; Patton, R.; Twaddle, N.; Doerge, D.; Churchwell, M.; Bryant, M.; et al. A two-year toxicology study of bisphenol A (BPA) in Sprague-Dawley rats: CLARITY-BPA core study results. Food Chem. Toxicol. 2019, 132, 110728.
- Heindel, J.J.; Newbold, R.R.; Bucher, J.R.; Camacho, L.; Delclos, K.B.; Lewis, S.M.; VanLandingham, M.; Churchwell, M.I.; Twaddle, N.C.; McLellen, M.; et al. NIEHS/FDA CLARITY-BPA research program update. Reprod. Toxicol. 2015, 58, 33–44.
- Dere, E.; Anderson, L.M.; Huse, S.M.; Spade, D.J.; McDonnell-Clark, E.; Madnick, S.J.; Hall, S.J.; Camacho, L.; Lewis, S.M.; VanLandingham, M.M.; et al. Effects of continuous bisphenol A exposure from early gestation on 90-day old rat testes function and sperm molecular profiles: A CLARITY-BPA consortium study. Toxicol. Appl. Pharmacol. 2018, 347, 1–9.
- Morrissey, R.E.; George, J.D.; Price, C.J.; Tyl, R.W.; Marr, M.C.; Kimmel, C.A. The Developmental Toxicity of Bisphenol A in Rats and Mice. Toxicol. Sci. 1987, 8, 571–582.
- Saunders, P.T.K.; Majdic, G.; Parte, P.; Millar, M.R.; Fisher, J.S.; Turner, K.J.; Sharpe, R.M. Fetal and Perinatal Influence of Xenoestrogens on Testis Gene Expression. Results Probl. Cell Differ. 1997, 424, 99–110.
- Hart, R.J.; Doherty, D.A.; Keelan, J.A.; Minaee, N.S.; Thorstensen, E.B.; Dickinson, J.E.; Pennell, C.E.; Newnham, J.P.; McLachlan, R.; Norman, R.J.; et al. The impact of antenatal Bisphenol A exposure on male reproductive function at 20–22 years of age. Reprod. Biomed. Online 2018, 36, 340–347.
- Vitku, J.; Chlupacova, T.; Sosvorova, L.; Hampl, R.; Hill, M.; Heracek, J.; Bicikova, M.; Starka, L. Development and validation of LC–MS/MS method for quantification of bisphenol A and estrogens in human plasma and seminal fluid. Talanta 2015, 140, 62–67.
- Zhang, T.; Sun, H.; Kannan, K. Blood and Urinary Bisphenol A Concentrations in Children, Adults, and Pregnant Women from China: Partitioning between Blood and Urine and Maternal and Fetal Cord Blood. Environ. Sci. Technol. 2013, 47, 4686–4694.
- Lan, H.-C.; Wu, K.-Y.; Lin, I.-W.; Yang, Z.-J.; Chang, A.-A.; Hu, M.-C. Bisphenol A disrupts steroidogenesis and induces a sex hormone imbalance through c-Jun phosphorylation in Leydig cells. Chemosphere 2017, 185, 237–246.
- Akingbemi, B.T.; Sottas, C.M.; Koulova, A.I.; Klinefelter, G.R.; Hardy, M.P. Inhibition of Testicular Steroidogenesis by the Xenoestrogen Bisphenol A Is Associated with Reduced Pituitary Luteinizing Hormone Secretion and Decreased Steroidogenic Enzyme Gene Expression in Rat Leydig Cells. Endocrinology 2004, 145, 592–603.
- Xi, W.; Lee, C.; Yeung, W.; Giesy, J.P.; Wong, M.; Zhang, X.; Hecker, M.; Wong, C.K. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus–pituitary–gonadal axis of CD-1 mice. Reprod. Toxicol. 2011, 31, 409–417.
- Hong, J.; Chen, F.; Wang, X.; Bai, Y.; Zhou, R.; Li, Y.; Chen, L.; Bai, Y. Exposure of preimplantation embryos to low-dose bisphenol A impairs testes development and suppresses histone acetylation of StAR promoter to reduce production of testosterone in mice. Mol. Cell. Endocrinol. 2016, 427, 101–111.
- Ben Maamar, M.; Lesné, L.; Desdoits-Lethimonier, C.; Coiffec, I.; Lassurguère, J.; Lavoué, V.; Deceuninck, Y.; Antignac, J.-P.; Le Bizec, B.; Perdu, E.; et al. An Investigation of the Endocrine-Disruptive Effects of Bisphenol A in Human and Rat Fetal Testes. PLoS ONE 2015, 10, e0117226.
- Lv, Y.; Li, L.; Fang, Y.; Chen, P.; Wu, S.; Chen, X.; Ni, C.; Zhu, Q.; Huang, T.; Lian, Q.; et al. In utero exposure to bisphenol A disrupts fetal testis development in rats. Environ. Pollut. 2019, 246, 217–224.
- Hanaoka, T.; Kawamura, N.; Hara, K.; Tsugane, S. Urinary bisphenol A and plasma hormone concentrations in male workers exposed to bisphenol A diglycidyl ether and mixed organic solvents. Occup. Environ. Med. 2002, 59, 625–628.
- Meeker, J.D.; Yang, T.; Ye, X.; Calafat, A.M.; Hauser, R. Urinary Concentrations of Parabens and Serum Hormone Levels, Semen Quality Parameters, and Sperm DNA Damage. Environ. Health Perspect. 2011, 119, 252–257.
- Mendiola, J.; Jørgensen, N.; Andersson, A.M.; Calafat, A.M.; Ye, X.; Redmon, J.B.; Drobnis, E.Z.; Wang, C.; Sparks, A.; Thurston, S.W.; et al. Are environmental levels of bisphenol A associated with reproductive function in fertile men? Environ. Health Perspect. 2010, 118, 1286–1291.
- Galloway, T.; Cipelli, R.; Guralnik, J.; Ferrucci, L.; Bandinelli, S.; Corsi, A.M.; Money, C.; McCormack, P.; Melzer, D. Daily Bisphenol A Excretion and Associations with Sex Hormone Concentrations: Results from the InCHIANTI Adult Population Study. Environ. Health Perspect. 2010, 118, 1603–1608.
- Lassen, T.H.; Frederiksen, H.; Jensen, T.K.; Petersen, J.H.; Joensen, U.N.; Main, K.M.; Skakkebaek, N.E.; Juul, A.; Jørgensen, N.; Andersson, A.-M. Urinary Bisphenol A Levels in Young Men: Association with Reproductive Hormones and Semen Quality. Environ. Health Perspect. 2014, 122, 478–484.
- Jensen, T.K.; Andersson, A.M.; Jørgensen, N.; Andersen, A.G.; Carlsen, E.; Skakkebæk, N.E. Body mass index in relation to semen quality and reproductive hormonesamong 1,558 Danish men. Fertil. Steril. 2004, 82, 863–870.
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198.
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276.
- Brigelius-Flohé, R. Glutathione peroxidases and redox-regulated transcription factors. Biol. Chem. 2006, 387, 1329–1335.
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84.
- Powell, S.R. The Antioxidant Properties of Zinc. J. Nutr. 2000, 130, 1447S–1454S.
- Prasad, A.S. Zinc is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health. Front. Nutr. 2014, 1, 14.
- Johnson, M.A.; Fischer, J.G.; Kays, S.E. Is copper an antioxidant nutrient? Crit. Rev. Food Sci. Nutr. 1992, 32, 1–31.
- Sahu, C.; Charaya, A.; Singla, S.; Dwivedi, D.K.; Jena, G. Zinc deficient diet increases the toxicity of bisphenol A in rat testis. J. Biochem. Mol. Toxicol. 2020, 34.
- Rhee, S.G. Overview on Peroxiredoxin. Mol. Cells 2016, 39, 1–5.
- Masutani, H.; Ueda, S.; Yodoi, J. The thioredoxin system in retroviral infection and apoptosis. Cell Death Differ. 2005, 12, 991–998.
- Akarca-Dizakar, S.Ö.; Erdoğan, D.; Peker, T.; Coşkun Akçay, N.; Türkoğlu, I.; Eşmekaya, M.A.; Ömeroğlu, S. Effects of co-administered melatonin, fructose and bisphenol A (BPA) on rat epididymis and sperm characteristics. Biotech. Histochem. 2020, 95, 18–26.
- Olukole, S.G.; Lanipekun, D.O.; Ola-Davies, E.O.; Oke, B.O. Maternal exposure to environmentally relevant doses of bisphenol A causes reproductive dysfunction in F1 adult male rats: Protective role of melatonin. Environ. Sci. Pollut. Res. 2019, 26, 28940–28950.
- Gules, O.; Yildiz, M.; Naseer, Z.; Tatar, M. Effects of folic acid on testicular toxicity induced by bisphenol-A in male Wistar rats. Biotech. Histochem. 2018, 94, 26–35.
- Rahman, S.; Kang, K.-H.; Arifuzzaman, S.; Pang, W.-K.; Ryu, D.-Y.; Song, W.-H.; Park, Y.-J.; Pang, M.-G. Effect of antioxidants on BPA-induced stress on sperm function in a mouse model. Sci. Rep. 2019, 9, 1–10.
- Aikawa, H.; Koyama, S.; Matsuda, M.; Nakahashi, K.; Akazome, Y.; Mori, T. Relief effect of vitamin A on the decreased motility of sperm and the increased incidence of malformed sperm in mice exposed neonatally to bisphenol A. Cell Tissue Res. 2004, 315, 119–124.
- Jiang, Z.; Wang, J.; Li, X.; Zhang, X. Echinacoside and Cistanche tubulosa (Schenk) R. wight ameliorate bisphenol A-induced testicular and sperm damage in rats through gonad axis regulated steroidogenic enzymes. J. Ethnopharmacol. 2016, 193, 321–328.
- Alboghobeish, S.; Mahdavinia, M.; Zeidooni, L.; Samimi, A.; Oroojan, A.A.; Alizadeh, S.; Dehghani, M.A.; Ahangarpour, A.; Khorsandi, L. Efficiency of naringin against reproductive toxicity and testicular damages induced by bisphenol A in rats. Iran. J. Basic Med. Sci. 2019, 22, 315–523.
- Jahan, S.; Ain, Q.U.; Ullah, H. Therapeutic effects of quercetin against bisphenol A induced testicular damage in male Sprague Dawley rats. Syst. Biol. Reprod. Med. 2016, 62, 114–124.
- Kaur, S.; Sadwal, S. Studies on the phytomodulatory potential of fenugreek (Trigonella foenum-graecum) on bisphenol-A induced testicular damage in mice. Andrology 2019, 52, e13492.
- Park, B.; Kwon, J.E.; Cho, S.M.; Kim, C.W.; Lee, D.E.; Koo, Y.T.; Lee, S.H.; Lee, H.M.; Kang, S.C. Protective effect of Lespedeza cuneata ethanol extract on Bisphenol A-induced testicular dysfunction in vivo and in vitro. Biomed. Pharmacother. 2018, 102, 76–85.
- Tamilselvan, P.; Bharathiraja, K.; Vijayaprakash, S.; Balasubramanian, M.P. Protective role of lycopene on bisphenol A induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Int. J. Pharm. Biol. Sci. 2013, 4, 131–143.
- Rezaee-Tazangi, F.; Zeidooni, L.; Rafiee, Z.; Fakhredini, F.; Kalantari, H.; Alidadi, H.; Khorsandi, L. Taurine effects on Bisphenol A-induced oxidative stress in the mouse testicular mitochondria and sperm motility. JBRA Assist. Reprod. 2020, 1–8.
- Minamiyama, Y.; Ichikawa, H.; Takemura, S.; Kusunoki, H.; Naito, Y.; Yoshikawa, T. Generation of reactive oxygen species in sperms of rats as an earlier marker for evaluating the toxicity of endocrine-disrupting chemicals. Free Radic. Res. 2010, 44, 1398–1406.
