The human gut microflora comprises over 1000 species and more than 7000 strains [23], representing 1013–1014 bacterial cells, which is ten times more numerous than other cells. Healthy gut microbiota is mainly composed of the phyla Firmicutes and Bacteroidetes, representing around 90% of the human gut flora, followed by Actinobacteria, Verrucomicrobia, and Proteobacteria.
- Omega-3 PUFA
- cardiovascular diseases
- microbiota
- dysbiosis
1. Cardioprotective roles of omega-3 PUFAs
Omega-3 polyunsaturated fatty acids (PUFAs) are often associated with cardio-protective effects. Recent studies suggest that omega-3 PUFAs could attenuate the deleterious impact of a dysbiosis by acting on gut microbiota, a common characteristic observed in patients with cardiovascular diseases. In this review, we will discuss the potential role of omega-3 PUFAs on the microbiota and their possible effects on cardiovascular diseases.
Large controlled randomized trials comprising more than 32,000 participants provided evidence of a major reduction of cardiovascular events by omega-3 PUFA supplements [1][2][3]. However, these beneficial effects are not reported universally. For example, the OMEGA trial indicated that the effects of 1 g/day of omega-3-acid ethyl esters-90 did not further reduce sudden cardiac death and other clinical events [4]. Probably one of the most important analyses of omega-3 PUFA supplementation in cardiovascular disease involving 68,680 patients indicates that omega-3 PUFA supplementation is not associated with a lower risk of all-cause mortality, cardiac death, sudden death, myocardial infarction (MI), and stroke [5]. The results of the ORIGIN study (12,536 high-risk patients with or at risk of diabetes) reveal that omega-3 PUFA treatment does not reduce the total mortality, cardiovascular mortality or any cardiovascular events compared to the placebo [6]. The difference between beneficial and no effect studies are still a matter of debate, but the dose or the ratio between the different omega-3 PUFA could be an issue. More recently, the addition of 4 g/day of icosapent ethyl demonstrated a reduction of the ischemic events in patients statin-treated high-risk patients [7], suggesting that a higher dosage or a unique omega-3 PUFA could be more effective. The latter point has been observed in our experimental study, demonstrating that the addition of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in the diet did not afford protection in contrast to each omega-3 PUFA alone at the same dose [8].
In experimental studies, results indicated a reduction of infarct size in many species fed with a multiple long-chain omega 3 PUFA [9][10][11] or with only one [12][13]. Overall, it seems that there is a large consensus in favor of reduced infarct size with omega-3 PUFA in animal experiments.
Until the present day, there were a lot of mechanisms by which omega-3 PUFA could exert cardio-protective effects. The anti-inflammatory properties of omega-3 PUFA are certainly the first one that we have considered. These properties could be related to their incorporation in cell membrane phospholipids, largely at the expense of AA (omega-6 PUFA), which is pro-inflammatory [14]. Other possibilities have been uncovered with the identification of G-protein coupled receptors (GPCR) that interact with fatty acids (GPR43, GPR120) [15][16]. For instance, DHA interacts with GPR120 and could inhibit iΚB kinase as well as the production of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNFα). DHA and EPA may also inhibit NF-κB activity by the interaction with PPARγ or interference with early events before NF-κB activation [17].
We also observed that a high omega-3 PUFA diet is cardio-protective via a mechanism involving Akt activation [11], an enzyme identified to be part of the key biochemical pathway component the reperfusion injury salvage kinase (RISK) [18]. When activated at the onset of the reperfusion, these kinases confer cardio-protection by mPTP opening inhibition [19]. DHA could also inhibit the opening of the mPTP and result in a reduction of infarct size [8] by an unknown mechanism.
In addition to the direct effect of omega-3 PUFA, we must also consider the metabolites involved in the resolution phase of inflammation known as resolvins (Rv). We observed that RvD1 administration before the onset of reperfusion reduced myocardial infarct size in a porcine model [20]. We also observed that when there is inhibition of the main enzymes involved in DHA transformation to RvD1 (COX-2 and 15-LOX), plasma RvD1 concentrations are reduced and the cardio-protection is abolished [21]. Similarly, Keyes et al. reported that RvE1 administration in a rat model of MI significantly reduces infarct size and increases Akt and Erk activity [22]. This indicates the potential role of these metabolites in the cardio-protection observed with omega-3 PUFAs.
2. Microbiota and Dysbiosis
The human gut microflora comprises over 1000 species and more than 7000 strains [23], representing 1013–1014 bacterial cells, which is ten times more numerous than other cells. Healthy gut microbiota is mainly composed of the phyla Firmicutes and Bacteroidetes, representing around 90% of the human gut flora, followed by Actinobacteria, Verrucomicrobia, and Proteobacteria. The large intestine hosts over 70% of all microbes in the human body. Pathogens, such as Campylobacter jejuni, Salmonella enterica, Vibrio cholera, and Escherichia coli can also be found, but in low numbers. While microbiota composition varies between healthy individuals in terms of different taxa proportions and rapid bacterial alterations are observed in humans, their magnitude is modest [24].
Dysbiosis is frequently observed in cardiovascular diseases patients [25][26]. Dysbiosis is an “imbalance” in the gut microbial community. This imbalance could be due to the gain or loss of community members or changes in the relative abundance of microbes. In addition to obesity [27], metabolic syndrome [28], and type 2 diabetes [29], evidence suggest that diet can also induce dysbiosis [30], which is often associated with an increase in inflammation [31][32].
Different diets can induce dysbiosis, whose reported changes in the microbiota differ from diet to diet. For example, a diet rich in complex carbohydrates increases Bifidobacteria [33], while a high-fat and high sugar diet results in an increase in Clostridium innocuum, Catenibacterium mitsuokai, and Enterococcus spp. [34]. Elevated diet in fat or carbohydrate in humans is associated with a decrease in Bacteroidetes and an increase in Firmicutes [35], which is also reported in animals [36][37]. In humans, an omega-3-PUFA rich diet (600 mg daily) for 14 days increased the abundance of several bacteria producing the short-chain fatty acid (SCFA) butyrate [38], known to have anti-inflammatory effects [39][40]. A 14 day washout reverses these effects. Similarly, in mice, fish oil treatment for 15 days elicits significant gut microbiota changes. This result indicates that diets rapidly affect microbiota [41]. A high-fat diet causes dysbiosis by increasing the ratio of Gram−/Gram+ bacteria [42]. The increase in Gram− bacteria, of which γ-Proteobacteria is a part, correlates with an increase in lipopolysaccharide (LPS), which promotes inflammation. In turn, this inflammation contributes to a modification of the microbiota, favoring the proliferation of other bacteria of an inflammatory nature [43]. This, therefore, suggests that a diet rich in ω-3 would be beneficial for health and that it helps restore the balance of the microbiota (Figure 1).
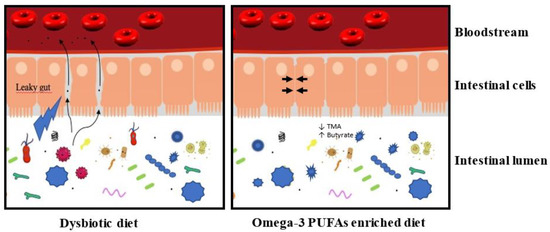
Figure 1. A dysbiotic diet disturbs the composition of the intestinal microbiota and affects the integrity of the intestinal barrier. In these conditions, the intestinal lumen’s content could transfer to the bloodstream and induce a low-grade inflammation. This low-grade inflammation contributes negatively to the size of the myocardial infarction and the development of heart failure. An enriched omega-3 PUFA diet could maintain a healthy microbiota and preserve the integrity of the intestinal barrier (→ ←). This microbiota is associated with a decrease in TMA production and an increase of butyrate (see text for details). In these conditions, the enriched omega-3 PUFA diet prevents the induction of low-grade inflammation.
Along with nutritional influences, studies have shown that sex [44][45][46][47][48][49] also modulates microbiota composition. For example, in the same experimental conditions, males generally show more microbiota changes than females [47]. According to a recent study, male mice are more sensitive to DHA than females, exhibiting more significant changes in their gut microbiota [44]. However, some studies indicate that adding two omega-3 PUFAs (EPA and DHA) induced significant changes in female mice microbiota [50], while in other studies, males and females showed similar variations [41]. These data underscore the importance of continuing studies on the impact of sex to better understand the potential sex-specific mechanisms underlying microbiota’s influence on health.
The gut microbiota participates in several physiological functions for maintaining health, including its involvement in the catabolic pathways to produce SCFA and cometabolites such as ammonia, phenols, indoles, various amines and thiols [51]. The gut microbiota also contributes to regulating the intestinal mucosal barrier, the control of nutrient uptake and metabolism, the maturation of immunologic tissues, and the prevention of the propagation of pathogenic microorganisms [52][53][54][55][56]. Thus, the regulation of these functions is essential to limit diseases, including cardiovascular diseases.
2.1. Myocardial Infarction and Microbiota
No direct evidence is presently available that demonstrates gut microbes influences on infarct size [57]. However, a mango-flavored Goodbelly juice, containing a probiotic, L. Plantarum 299 v, reduced the size of the myocardial infarct [58]. It is difficult to confirm that the positive effect is only related to the probiotic since other components are present. A study by Lam et al. indicates a link between gut microbiota metabolites and the severity of the myocardial infarction [59]. In another study, the data indicate that the microbiota’s richness is higher in the rat MI group than in the sham group at day seven after the onset of ischemia. This change parallels intestinal barrier impairment documented by reducing the occludin (a tight junction protein) and the Chiu pathological scores of mucosal injuries [60].
Interestingly, we also observed that MI induces a change in intestinal barrier integrity 14 days post-MI in our rat ischemia/reperfusion model [61]. Overall, data suggest a link between the gut microbiota and MI involving alteration of the gut barrier integrity.
2.2. Microbiota and Cardiovascular Diseases
Additionally, the evidence is accumulating for a role of the microbiota in the development of heart failure. According to the “gut hypothesis”, the decrease in cardiac output and the systemic congestion observed in this condition would promote intestinal mucosal ischemia and/or edema, as well as contributing to the translocation of bacteria, an increase in circulating endotoxins, thereby contributing to inflammation [62][63]. In patients with heart failure, an increase in intestinal permeability was also noted compared to controls [64].
While inflammation is well described in heart failure, several clinical studies hypothesized the benefit of reduced inflammation in heart failure have yielded rather disappointing results [65][66][67][68]. However, a recent study has revived this solution by targeting more patients with or without heart failure following a myocardial infarction benefiting from targeted anti-cytokine therapy [69].
Studies have shown that gut microbiota may also modulate the risk factors involved in developing MI and heart failure, such as atherosclerosis [70], hypertension [71], and obesity [72]. For instance, in Apoe−/− mice, treatment with ampicillin was used to decrease the number of bacteria, reduced low-density lipoprotein, very-low-density-lipoprotein cholesterol levels, and the atherosclerotic aortic lesion, compared with controls [73]. Gut microbiota was also reported to produce TMA, a precursor of trimethylamine-N-oxide (TMAO), which promotes atherosclerotic plaques [74].
Microbiota from spontaneously hypertensive rats (SHRs) transferred to Wistar Kyoto (WKY) rats results in a significant systolic blood pressure increase (26 mm Hg) compared with controls [75]. Others have reported that the Firmicutes to Bacteroidetes ratio is only increased in SHRs compared with pre-hypertensive SHRs or healthy rats [71], suggesting a link between microbiota composition and blood pressure.
Gut microbiota isolated from obese mice and transferred to germ-free recipients resulted in a 20% increase of total body fat than the transfer of gut microbiota isolated from lean mice [76], supporting a link between microbiota and obesity.
3. Potential Roles of Microbiota
The microbiota dysbiosis has already numerous impacts on cardiovascular diseases and could be involved at different levels, leading to increased myocardial damage. The following observations urge us to determine the impact of a “dysbiotic diet” on the ischemic myocardium.
The intestinal barrier regulates the absorption of nutrients, electrolytes, and water from the lumen. It prevents the passage of pathogenic microorganisms (or of their products) and toxic substances into the bloodstream [77]. Different features protect the barrier’s integrity, including a mucus layer and a monolayer of epithelial cells interconnected by tight junctions. The mucus layer contains immunoglobulin A and antimicrobial peptides that facilitate gastrointestinal transport and protection against bacterial invasion.
The tight junctions consist of complex protein structures (e.g., claudin, occludin, and tricullin) that form mechanical links between epithelial cells [78]. Gut microbes or their metabolites may modulate the intestinal barrier integrity: the exact mechanisms are unclear, but may include the following: (1) intestinal epithelial cell renewal [79] and cell death [80]; (2) activation of signalling pathways involved in barrier integrity (phosphatase, kinase) [81]; and (3) production of metabolites that reduce inflammation [82].
Compromised intestinal barrier integrity correlates with chronic, low-grade inflammation [82]. The translocation of the bacterial lipopolysaccharide (LPS) component of Gram-negative bacteria in the bloodstream could play a significant role [83]. LPS concentrations could be 10–50 times lower than in septicemia or infection, but could still be sufficient to evoke inflammation [84].
Gut microbiota could also affect inflammation levels by acting on the vagus nerve. Vagus nerve stimulation reduces infarct size [85][86][87][88] by a mechanism involving cholinergic activation’s anti-inflammatory properties. Recent studies show that the microbiota’s composition could modulate the vagus nerve activation by an undefined mechanism [89][90][91].
Microbiota could also affect infarct size by producing SCFAs, through their anti-inflammatory properties [92][93]. Non-digested polysaccharides are fermented by gut microbes, generating SCFAs, mostly acetate, propionate, and butyrate [94][95]. These metabolites present well-characterized, anti-inflammatory properties, and modulate cellular functions through G-protein coupled receptors (GPR41, GPR43, and GPR109A) or inhibiting histone deacetylases [96][97][98][99].
The gut microbiota metabolizes trimethylamine (TMA)-containing compounds (choline, phosphatidylcholine, and carnitine) to TMA, a precursor of trimethylamine-N-oxide (TMAO) produced by hepatic cells [100]. Several publications have reported that TMAO heightens cardiovascular risk by promoting atherosclerotic lesions [74] or platelet activation [101]. TMAO suppresses reverse cholesterol transport [102] and up-regulates pro-atherogenic scavenger receptors [103].
Accumulating experimental data suggest the gut microbiota participates in different pathological states where inflammation is involved, which could impact infarct size. MI is an inflammatory pathology involving neutrophil accumulation [104] and the production of pro-inflammatory substances, such as cytokines [105][106] or arachidonic acid derivatives [107][108][109]. Infarct size reduction may also be caused by the attenuation of neutrophil accumulation [110], injection of anti-inflammatory molecules [111][112] or resolvins [20][21][22], which participate in the resolution phase of inflammation. These observations urge us to determine the impact of a “dysbiotic diet” on the ischemic myocardium.
Moreover, in addition to the impact on myocardial infarct size, microbiota could also interfere with mechanisms that alter the healing of infarcted myocardium and heart failure development.
TMAO and butyrate are among molecules released by the microbiota that are suspected of impairing myocardial healing or precipitated heart failure. Some even suggest that the TMAO would be a predictor of mortality in the case of heart failure. While the mechanism remains speculative, most authors agree that the inflammation associated with the presence of TMAO is linked to the increased production of pro-inflammatory cytokines such as TNFα, IL-6, and IL-1β [113] or the activation of NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome and NF-κB. Also, TMAO could promote apoptosis [114]. This increase in the production of TMAO is associated with an augmentation of the bacteria that belong to the genus of Clostridium, Escherichia, and Proteus [115].
Others have reported that concentrations of butyrate in feces and plasma are lower in cases of heart failure [116]. An anti-inflammatory response to butyrate via NF-κB inhibition is reported in several in vitro and in vivo studies where a decrease in myeloperoxidase concentrations cyclooxygenase-2 and cytokines has been identified [117][118][119][120], with some effects related to the FFAR3 receptor. This reduction of butyrate is associated with a reduction in bacteria that belong to the genus of Roseburia, Faecalibacterium, and Eubacterium [121]. In these circumstances, a high omega-3 PUFA diet could attenuate dysbiosis as well as limit damage due to the microbiota.
In conclusion, the data suggest that a diet rich in omega-3 PUFAs affects the composition of the microbiota, thereby alleviating the dysbiosis seen in patients with cardiovascular disease and reducing the deleterious effects associated with dysbiotic microbiota. However, it remains essential to know whether this effect is observable, regardless of gender and whether age alters this relationship or not. Overall, this new avenue of interventions is exciting but further studies are needed to have a better understanding of omega-3 PUFAs on the modulation of microbiota and these effects on cardiovascular diseases.
References
- Burr, M.L.; Fehily, A.M.; Gilbert, J.F.; Rogers, S.; Holliday, R.M.; Sweetnam, P.M.; Elwood, P.C.; Deadman, N.M. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial (DART). Lancet 1989, 2, 757–761.
- Marchioli, R.; Barzi, F.; Bomba, E.; Chieffo, C.; Di Gregorio, D.; Di Mascio, R.; Franzosi, M.G.; Geraci, E.; Levantesi, G.; Maggioni, A.P.; et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: Time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002, 105, 1897–1903.
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098.
- Rauch, B.; Schiele, R.; Schneider, S.; Diller, F.; Victor, N.; Gohlke, H.; Gottwik, M.; Steinbeck, G.; Del Castillo, U.; Sack, R.; et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 2010, 122, 2152–2159.
- Rizos, E.C.; Ntzani, E.E.; Bika, E.; Kostapanos, M.S.; Elisaf, M.S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012, 308, 1024–1033.
- Bosch, J.; Gerstein, H.C.; Dagenais, G.R.; Diaz, R.; Dyal, L.; Jung, H.; Maggiono, A.P.; Probstfield, J.; Ramachandran, A.; Riddle, M.C.; et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med. 2012, 367, 309–318.
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Effects of Icosapent Ethyl on Total Ischemic Events: From REDUCE-IT. J. Am. Coll. Cardiol. 2019, 73, 2791–2802.
- Madingou, N.; Gilbert, K.; Tomaro, L.; Prud’homme Touchette, C.; Trudeau, F.; Fortin, S.; Rousseau, G. Comparison of the effects of EPA and DHA alone or in combination in a murine model of myocardial infarction. Prostaglandins Leukot Essent Fat. Acids 2016, 111, 11–16.
- Zhu, B.Q.; Sievers, R.E.; Sun, Y.P.; Morse-Fisher, N.; Parmley, W.W.; Wolfe, C.L. Is the reduction of myocardial infarct size by dietary fish oil the result of altered platelet function? Am. Heart J. 1994, 127 (Pt 1), 744–755.
- Yang, B.C.; Saldeen, T.G.; Bryant, J.L.; Nichols, W.W.; Mehta, J.L. Long-term dietary fish oil supplementation protects against ischemia-reperfusion-induced myocardial dysfunction in isolated rat hearts. Am. Heart J. 1993, 126, 1287–1292.
- Rondeau, I.; Picard, S.; Bah, T.M.; Roy, L.; Godbout, R.; Rousseau, G. Effects of different dietary omega-6/3 polyunsaturated fatty acids ratios on infarct size and the limbic system after myocardial infarction. Can. J Physiol. Pharm. 2011, 89, 169–176.
- Ogita, H.; Node, K.; Asanuma, H.; Sanada, S.; Takashima, S.; Minamino, T.; Soma, M.; Kim, J.; Hori, M.; Kitakaze, M. Eicosapentaenoic acid reduces myocardial injury induced by ischemia and reperfusion in rabbit hearts. J. Cardiovasc. Pharm. 2003, 41, 964–969.
- Xiao, Y.F.; Sigg, D.C.; Ujhelyi, M.R.; Wilhelm, J.J.; Richardson, E.S.; Iaizzo, P.A. Pericardial delivery of omega-3 fatty acid: A novel approach to reducing myocardial infarct sizes and arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2212–H2218.
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. Maywood 2008, 233, 674–688.
- Miyauchi, S.; Hirasawa, A.; Iga, T.; Liu, N.; Itsubo, C.; Sadakane, K.; Hara, T.; Tsujimoto, G. Distribution and regulation of protein expression of the free fatty acid receptor GPR120. Naunyn. Schmiedebergs Arch. Pharm. 2009, 379, 427–434.
- Cornall, L.M.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Diet-induced obesity up-regulates the abundance of GPR43 and GPR120 in a tissue specific manner. Cell Physiol. Biochem. 2011, 28, 949–958.
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662.
- Hausenloy, D.J.; Yellon, D.M. New directions for protecting the heart against ischaemia-reperfusion injury: Targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc. Res. 2004, 61, 448–460.
- Hausenloy, D.; Wynne, A.; Duchen, M.; Yellon, D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation 2004, 109, 1714–1717.
- Tran Quang, T.; Gosselin, A.-A.; Bourque-Riel, V.; Gilbert, K.; Charron, T.; Rousseau, G. Effect of Resolvin D1 on experimental myocardial infarction. Exp. Clin. Cardiol. 2014, 20, 6704–6712.
- Gilbert, K.; Malick, M.; Madingou, N.; Touchette, C.; Bourque-Riel, V.; Tomaro, L.; Rousseau, G. Metabolites derived from omega-3 polyunsaturated fatty acids are important for cardioprotection. Eur. J. Pharm. 2015, 769, 147–153.
- Keyes, K.T.; Ye, Y.; Lin, Y.; Zhang, C.; Perez-Polo, J.R.; Gjorstrup, P.; Birnbaum, Y. Resolvin E1 protects the rat heart against reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H153–H164.
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-gut-microbe communication in health and disease. Front. Physiol. 2011, 2, 94.
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108.
- Aryal, S.; Alimadadi, A.; Manandhar, I.; Joe, B.; Cheng, X. Machine Learning Strategy for Gut Microbiome-Based Diagnostic Screening of Cardiovascular Disease. Hypertension 2020, 76, 1555–1562.
- Suganya, K.; Son, T.; Kim, K.W.; Koo, B.S. Impact of gut microbiota: How it could play roles beyond the digestive system on development of cardiovascular and renal diseases. Microb. Pathog. 2020, 104583.
- Lopez-Moreno, A.; Suarez, A.; Avanzi, C.; Monteoliva-Sanchez, M.; Aguilera, M. Probiotic Strains and Intervention Total Doses for Modulating Obesity-Related Microbiota Dysbiosis: A Systematic Review and Meta-analysis. Nutrients 2020, 12, 1921.
- Bier, A.; Khasbab, R.; Haberman, Y.; Braun, T.; Hadar, R.; Sosnovski, K.; Amir, A.; Leibowitz, A.; Grossman, E. Antibiotic Treatment Does Not Ameliorate the Metabolic Changes in Rats Presenting Dysbiosis After Consuming a High Fructose Diet. Nutrients 2020, 12, 203.
- Wang, G.; Si, Q.; Yang, S.; Jiao, T.; Zhu, H.; Tian, P.; Wang, L.; Li, X.; Gong, L.; Zhao, J.; et al. Lactic acid bacteria reduce diabetes symptoms in mice by alleviating gut microbiota dysbiosis and inflammation in different manners. Food Funct. 2020, 11, 5898–5914.
- Brown, K.; DeCoffe, D.; Molcan, E.; Gibson, D.L. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients 2012, 4, 1095–1119.
- Singh, V.; Roth, S.; Llovera, G.; Sadler, R.; Garzetti, D.; Stecher, B.; Dichgans, M.; Liesz, A. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J. Neurosci. 2016, 36, 7428–7440.
- Tran, H.Q.; Bretin, A.; Adeshirlarijaney, A.; Yeoh, B.S.; Vijay-Kumar, M.; Zou, J.; Denning, T.L.; Chassaing, B.; Gewirtz, A.T. “Western Diet”-Induced Adipose Inflammation Requires a Complex Gut Microbiota. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 313–333.
- Pokusaeva, K.; Fitzgerald, G.F.; van Sinderen, D. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011, 6, 285–306.
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6ra14.
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023.
- de La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest Liver Physiol. 2010, 299, G440–G448.
- Turnbaugh, P.J.; Backhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host. Microbe 2008, 3, 213–223.
- Noriega, B.S.; Sanchez-Gonzalez, M.A.; Salyakina, D.; Coffman, J. Understanding the Impact of Omega-3 Rich Diet on the Gut Microbiota. Case Rep. Med. 2016, 2016, 3089303.
- Riviere, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979.
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988.
- Yu, H.N.; Zhu, J.; Pan, W.S.; Shen, S.R.; Shan, W.G.; Das, U.N. Effects of fish oil with a high content of n-3 polyunsaturated fatty acids on mouse gut microbiota. Arch. Med. Res. 2014, 45, 195–202.
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267.
- Rigottier-Gois, L. Dysbiosis in inflammatory bowel diseases: The oxygen hypothesis. ISME J. 2013, 7, 1256–1261.
- Davis, D.J.; Hecht, P.M.; Jasarevic, E.; Beversdorf, D.Q.; Will, M.J.; Fritsche, K.; Gillespie, C.H. Sex-specific effects of docosahexaenoic acid (DHA) on the microbiome and behavior of socially-isolated mice. Brain Behav. Immun. 2017, 59, 38–48.
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.; Drake, T.A.; Lusis, A.J. Sex differences and hormonal effects on gut microbiota composition in mice. Gut. Microbes 2016, 7, 313–322.
- Jasarevic, E.; Morrison, K.E.; Bale, T.L. Sex differences in the gut microbiome-brain axis across the lifespan. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150122.
- Zhang, H.; Wang, Z.; Li, Y.; Han, J.; Cui, C.; Lu, C.; Zhou, J.; Cheong, L.; Li, Y.; Sun, T.; et al. Sex-Based Differences in Gut Microbiota Composition in Response to Tuna Oil and Algae Oil Supplementation in a D-galactose-Induced Aging Mouse Model. Front. Aging Neurosci. 2018, 10, 187.
- Markle, J.G.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013, 339, 1084–1088.
- Audet, M.C. Stress-induced disturbances along the gut microbiota-immune-brain axis and implications for mental health: Does sex matter? Front Neuroendocr. 2019, 54, 100772.
- Ghosh, S.; DeCoffe, D.; Brown, K.; Rajendiran, E.; Estaki, M.; Dai, C.; Yip, A.; Gibson, D.L. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS ONE 2013, 8, e55468.
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904.
- Savage, D.C. Associations of indigenous microorganisms with gastrointestinal mucosal epithelia. Am. J. Clin. Nutr. 1970, 23, 1495–1501.
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920.
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723.
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118.
- Li, M.; Wang, B.; Zhang, M.; Rantalainen, M.; Wang, S.; Zhou, H.; Zhang, Y.; Shen, J.; Pang, X.; Zhang, M.; et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc. Natl. Acad. Sci. USA 2008, 105, 2117–2122.
- Zununi Vahed, S.; Barzegari, A.; Zuluaga, M.; Letourneur, D.; Pavon-Djavid, G. Myocardial infarction and gut microbiota: An incidental connection. Pharm. Res. 2017, 129, 308–317.
- Lam, V.; Su, J.; Koprowski, S.; Hsu, A.; Tweddell, J.S.; Rafiee, P.; Gross, G.J.; Salzman, N.H.; Baker, J.E. Intestinal microbiota determine severity of myocardial infarction in rats. Faseb. J. 2012, 26, 1727–1735.
- Lam, V.; Su, J.; Hsu, A.; Gross, G.J.; Salzman, N.H.; Baker, J.E. Intestinal Microbial Metabolites Are Linked to Severity of Myocardial Infarction in Rats. PLoS ONE 2016, 11, e0160840.
- Wu, Z.X.; Li, S.F.; Chen, H.; Song, J.X.; Gao, Y.F.; Zhang, F.; Cao, C.F. The changes of gut microbiota after acute myocardial infarction in rats. PLoS ONE 2017, 12, e0180717.
- Arseneault-Breard, J.; Rondeau, I.; Gilbert, K.; Girard, S.A.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr. 2012, 107, 1793–1799.
- Krack, A.; Richartz, B.M.; Gastmann, A.; Greim, K.; Lotze, U.; Anker, S.D.; Figulla, H.R. Studies on intragastric PCO2 at rest and during exercise as a marker of intestinal perfusion in patients with chronic heart failure. Eur. J. Heart Fail. 2004, 6, 403–407.
- Sandek, A.; Bauditz, J.; Swidsinski, A.; Buhner, S.; Weber-Eibel, J.; von Haehling, S.; Schroedl, W.; Karhausen, T.; Doehner, W.; Rauchhaus, M.; et al. Altered intestinal function in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007, 50, 1561–1569.
- Pasini, E.; Aquilani, R.; Testa, C.; Baiardi, P.; Angioletti, S.; Boschi, F.; Verri, M.; Dioguardi, F. Pathogenic Gut Flora in Patients With Chronic Heart Failure. JACC Heart Fail. 2016, 4, 220–227.
- Mann, D.L. Innate immunity and the failing heart: The cytokine hypothesis revisited. Circ. Res. 2015, 116, 1254–1268.
- Frantz, S.; Falcao-Pires, I.; Balligand, J.L.; Bauersachs, J.; Brutsaert, D.; Ciccarelli, M.; Dawson, D.; de Windt, L.J.; Giacca, M.; Hamdani, N.; et al. The innate immune system in chronic cardiomyopathy: A European Society of Cardiology (ESC) scientific statement from the Working Group on Myocardial Function of the ESC. Eur. J. Heart Fail. 2018, 20, 445–459.
- Dick, S.A.; Epelman, S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circ. Res. 2016, 119, 159–176.
- Van Linthout, S.; Tschöpe, C. Inflammation—Cause or Consequence of Heart Failure or Both? Curr. Heart Fail. Rep. 2017, 14, 251–265.
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271.
- Jonsson, A.L.; Backhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol 2017, 14, 79–87.
- Santisteban, M.M.; Qi, Y.; Zubcevic, J.; Kim, S.; Yang, T.; Shenoy, V.; Cole-Jeffrey, C.T.; Lobaton, G.O.; Stewart, D.C.; Rubiano, A.; et al. Hypertension-Linked Pathophysiological Alterations in the Gut. Circ. Res. 2017, 120, 312–323.
- de Groot, P.F.; Frissen, M.N.; de Clercq, N.C.; Nieuwdorp, M. Fecal microbiota transplantation in metabolic syndrome: History, present and future. Gut. Microbes 2017, 8, 253–267.
- Rune, I.; Rolin, B.; Larsen, C.; Nielsen, D.S.; Kanter, J.E.; Bornfeldt, K.E.; Lykkesfeldt, J.; Buschard, K.; Kirk, R.K.; Christoffersen, B.; et al. Modulating the Gut Microbiota Improves Glucose Tolerance, Lipoprotein Profile and Atherosclerotic Plaque Development in ApoE-Deficient Mice. PLoS ONE 2016, 11, e0146439.
- Troseid, M.; Hov, J.R.; Nestvold, T.K.; Thoresen, H.; Berge, R.K.; Svardal, A.; Lappegard, K.T. Major Increase in Microbiota-Dependent Proatherogenic Metabolite TMAO One Year After Bariatric Surgery. Metab. Syndr. Relat. Disord. 2016, 14, 197–201.
- Adnan, S.; Nelson, J.W.; Ajami, N.J.; Venna, V.R.; Petrosino, J.F.; Bryan, R.M., Jr.; Durgan, D.J. Alterations in the gut microbiota can elicit hypertension in rats. Physiol. Genom. 2017, 49, 96–104.
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031.
- Farhadi, A.; Banan, A.; Fields, J.; Keshavarzian, A. Intestinal barrier: An interface between health and disease. J. Gastroenterol. Hepatol. 2003, 18, 479–497.
- Dorfel, M.J.; Huber, O. Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. J. Biomed. Biotechnol 2012, 2012, 807356.
- Abrams, G.D.; Bauer, H.; Sprinz, H. Influence of the normal flora on mucosal morphology and cellular renewal in the ileum. A comparison of germ-free and conventional mice. Lab. Invest. 1963, 12, 355–364.
- Malick, M.; Gilbert, K.; Daniel, J.; Arseneault-Breard, J.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Vagotomy prevents the effect of probiotics on caspase activity in a model of postmyocardial infarction depression. Neurogastroenterol. Motil. 2015, 27, 663–671.
- Cario, E.; Gerken, G.; Podolsky, D.K. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 2004, 127, 224–238.
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392.
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772.
- Reinhardt, C.; Reigstad, C.S.; Backhed, F. Intestinal microbiota during infancy and its implications for obesity. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 249–256.
- Buchholz, B.; Donato, M.; Perez, V.; Deutsch, A.C.R.; Hocht, C.; Del Mauro, J.S.; Rodriguez, M.; Gelpi, R.J. Changes in the loading conditions induced by vagal stimulation modify the myocardial infarct size through sympathetic-parasympathetic interactions. Pflug. Arch. 2015, 467, 1509–1522.
- Shinlapawittayatorn, K.; Chinda, K.; Palee, S.; Surinkaew, S.; Kumfu, S.; Kumphune, S.; Chattipakorn, S.; KenKnight, B.H.; Chattipakorn, N. Vagus nerve stimulation initiated late during ischemia, but not reperfusion, exerts cardioprotection via amelioration of cardiac mitochondrial dysfunction. Heart Rhythm 2014, 11, 2278–2287.
- Uitterdijk, A.; Yetgin, T.; te Lintel Hekkert, M.; Sneep, S.; Krabbendam-Peters, I.; van Beusekom, H.M.; Fischer, T.M.; Cornelussen, R.N.; Manintveld, O.C.; Merkus, D.; et al. Vagal nerve stimulation started just prior to reperfusion limits infarct size and no-reflow. Basic Res. Cardiol. 2015, 110, 508.
- Zhang, R.; Wugeti, N.; Sun, J.; Yan, H.; Guo, Y.; Zhang, L.; Ma, M.; Guo, X.; Jiao, C.; Xu, W.; et al. Effects of vagus nerve stimulation via cholinergic anti-inflammatory pathway activation on myocardial ischemia/reperfusion injury in canine. Int. J. Clin. Exp. Med. 2014, 7, 2615–2623.
- Vaughn, A.C.; Cooper, E.M.; DiLorenzo, P.M.; O’Loughlin, L.J.; Konkel, M.E.; Peters, J.H.; Hajnal, A.; Sen, T.; Lee, S.H.; de La Serre, C.B.; et al. Energy-dense diet triggers changes in gut microbiota, reorganization of gutbrain vagal communication and increases body fat accumulation. Acta. Neurobiol. Exp. 2017, 77, 18–30.
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. North. Am. 2017, 46, 77–89.
- Forsythe, P.; Bienenstock, J.; Kunze, W.A. Vagal pathways for microbiome-brain-gut axis communication. Adv. Exp. Med. Biol. 2014, 817, 115–133.
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J. Atheroscler. Thromb. 2017, 24, 660–672.
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876.
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166.
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119.
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734.
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286.
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573.
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252.
- Ussher, J.R.; Lopaschuk, G.D.; Arduini, A. Gut microbiota metabolism of L-carnitine and cardiovascular risk. Atherosclerosis 2013, 231, 456–461.
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124.
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63.
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585.
- de Lorgeril, M.; Rousseau, G.; Basmadjian, A.; St-Jean, G.; Tran, D.C.; Latour, J.G. Spacial and temporal profiles of neutrophil accumulation in the reperfused ischemic myocardium. Am. J. Cardiovasc. Pathol. 1990, 3, 143–154.
- Moro, C.; Jouan, M.G.; Rakotovao, A.; Toufektsian, M.C.; Ormezzano, O.; Nagy, N.; Tosaki, A.; de Leiris, J.; Boucher, F. Delayed expression of cytokines after reperfused myocardial infarction: Possible trigger for cardiac dysfunction and ventricular remodeling. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3014–H3019.
- Kosmala, W.; Przewlocka-Kosmala, M.; Mazurek, W. Proinflammatory cytokines and myocardial viability in patients after acute myocardial infarction. Int. J. Cardiol. 2005, 101, 449–456.
- Saito, T.; Rodger, I.W.; Shennib, H.; Hu, F.; Tayara, L.; Giaid, A. Cyclooxygenase-2 (COX-2) in acute myocardial infarction: Cellular expression and use of selective COX-2 inhibitor. Can. J. Physiol. Pharm. 2003, 81, 114–119.
- Carnieto, A., Jr.; Dourado, P.M.; Luz, P.L.; Chagas, A.C. Selective cyclooxygenase-2 inhibition protects against myocardial damage in experimental acute ischemia. Clin. Sao Paulo 2009, 64, 245–252.
- Takase, B.; Maruyama, T.; Kurita, A.; Uehata, A.; Nishioka, T.; Mizuno, K.; Nakamura, H.; Katsura, K.; Kanda, Y. Arachidonic acid metabolites in acute myocardial infarction. Angiology 1996, 47, 649–661.
- de Lorgeril, M.; Basmadjian, A.; Lavallee, M.; Clement, R.; Millette, D.; Rousseau, G.; Latour, J.G. Influence of leukopenia on collateral flow, reperfusion flow, reflow ventricular fibrillation, and infarct size in dogs. Am. Heart J. 1989, 117, 523–532.
- Jolly, S.R.; Lucchesi, B.R. Effect of BW755C in an occlusion-reperfusion model of ischemic myocardial injury. Am. Heart J. 1983, 106 (Pt 1), 8–13.
- Mullane, K.M.; Moncada, S. The salvage of ischaemic myocardium by BW755C in anaesthetised dogs. Prostaglandins 1982, 24, 255–266.
- Rohrmann, S.; Linseisen, J.; Allenspach, M.; von Eckardstein, A.; Muller, D. Plasma Concentrations of Trimethylamine-N-oxide Are Directly Associated with Dairy Food Consumption and Low-Grade Inflammation in a German Adult Population. J. Nutr 2016, 146, 283–289.
- Yang, G.; Lin, C.C.; Yang, Y.; Yuan, L.; Wang, P.; Wen, X.; Pan, M.H.; Zhao, H.; Ho, C.T.; Li, S. Nobiletin Prevents Trimethylamine Oxide-Induced Vascular Inflammation via Inhibition of the NF-κB/MAPK Pathways. J. Agric. Food Chem. 2019, 67, 6169–6176.
- Liu, Y.; Dai, M. Trimethylamine N-Oxide Generated by the Gut Microbiota Is Associated with Vascular Inflammation: New Insights into Atherosclerosis. Mediat. Inflamm. 2020, 2020, 4634172.
- Trøseid, M.; Andersen, G.; Broch, K.; Hov, J.R. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine 2020, 52, 102649.
- Elce, A.; Amato, F.; Zarrilli, F.; Calignano, A.; Troncone, R.; Castaldo, G.; Canani, R.B. Butyrate modulating effects on pro-inflammatory pathways in human intestinal epithelial cells. Benef. Microbes 2017, 8, 841–847.
- Andoh, A.; Bamba, T.; Sasaki, M. Physiological and anti-inflammatory roles of dietary fiber and butyrate in intestinal functions. Jpen. J. Parenter Enter. Nutr 1999, 23 (Suppl. 5), S70–S73.
- Segain, J.P.; Raingeard de la Blétière, D.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottière, H.M.; Galmiche, J.P. Butyrate inhibits inflammatory responses through NFkappaB inhibition: Implications for Crohn’s disease. Gut. 2000, 47, 397–403.
- Song, M.; Xia, B.; Li, J. Effects of topical treatment of sodium butyrate and 5-aminosalicylic acid on expression of trefoil factor 3, interleukin 1beta, and nuclear factor kappaB in trinitrobenzene sulphonic acid induced colitis in rats. Postgrad. Med. J. 2006, 82, 130–135.
- La Rosa, S.L.; Leth, M.L.; Michalak, L.; Hansen, M.E.; Pudlo, N.A.; Glowacki, R.; Pereira, G.; Workman, C.T.; Arntzen, M.O.; Pope, P.B.; et al. The human gut Firmicute Roseburia intestinalis is a primary degrader of dietary beta-mannans. Nat. Commun. 2019, 10, 905.
