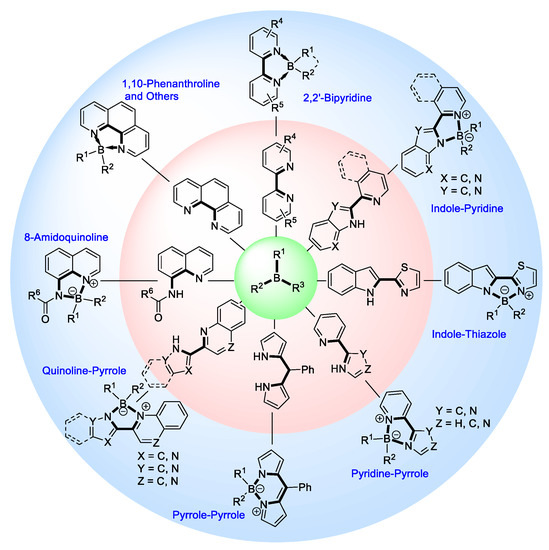
N,N′-chelate organoboron compounds have been successfully applied in bioimaging, organic light-emitting diodes (OLEDs), functional polymer, photocatalyst, electroluminescent (EL) devices, and other science and technology areas. However, the concise and efficient synthetic methods become more and more significant for material science, biomedical research, or other practical science. Here, we summarized the organoboron-N,N'-chelate derivatives and showed the different routes of their synthesis. Traditional methods to synthesize N,N'-chelate organoboron compounds were mainly using bidentate ligand containing nitrogen reacting with trivalent boron reagents. In this review, we described a series of bidentate ligands, such as bipyridine, 2-(pyridin-2-yl)-1H-indole, 2-(5-methyl-1H-pyrrol-2-yl)quinoline, N-(quinolin-8-yl)acetamide, 1,10-phenanthroline, and diketopyrrolopyrrole (DPP).
- organoboron
- NN′-chelate
- tetracoordinated
- fluorescent materials
1. Introduction
N,N′-chelate tetracoordinated organoboron compounds have been widely applied in various science and technology areas. For example, boron dipyrromethene derivatives (BODIPY) were developed in luminescent materials [1][2][3][4][5], dyes [6][7][8][9], photosensitizers [10][11][12][13][14], molecular switches [15][16][17], photodynamic therapy [18][19][20][21][22], molecular probes [23][24][25][26], and bioimaging [27][28][29]. In recent decades, N,N′-chelate compounds become a popular topic and have gradually attracted the attention of scientists. Four-coordinated organoboron compounds (BAr2 (N, N)) have interesting luminescent properties that can be modulated by various substituents in the N, N′-chelating framework [30]. More and more similar structures were explored and exhibited wonderful results in fluorescent materials [30][31], cell bioimaging (A1) [32], organic light-emitting diodes (OLEDs) (A2) [33], functional polymer (A3) [34], photocatalyst (A8) [35], electroluminescent (EL) devices [36], and so on, as shown in Figure 1. Herein, various traditional methods for the formation of N,N′-chelate tetracoordinated organoboron complexes were exhibited in this review. The main highlights include that (a) the diversified synthetic methods were provided, (b) readily available trivalent boron compounds were used as boron reagents, (c) various complex starting materials were designed as good bidentate ligands, and (d) these reactions showed a wide range of tetracoordinated organoboron complexes and excellent optical properties.
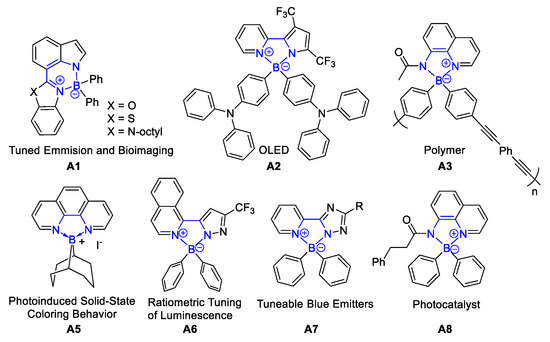
Figure 1. Application of tetracoordinate organoboron complexes in luminescent materials, organic light-emitting diode (OLED), functional polymer, and photocatalyst [30][31][32][33][34][35][36].
In recent years, the research on their syntheses falls into the following categories. At first, bipyridine derivatives could be used as bidentate ligand reacting with triphenylboron to form the desired products, as shown in Figure 2. The second, an indole connecting with pyridine derivatives (or other nitrogen heterocyclic molecule) reacted with triphenylboron to obtain the corresponding compounds, as shown in Figure 2. The third, pyridine attaching on pyrrole derivatives (or other nitrogen heterocyclic molecule) and trivalent boron could produce the fluorescent organoboron compounds, as shown in Figure 2. The fourth, a quinoline linking with pyrrole derivatives (or other nitrogen heterocyclic molecule) has been reported as a bidentate ligand to react with boron reagents. The different fluorescent compounds could be synthesized by N-(quinolin-8-yl)acetamide derivatives and boron reagents, as shown in Figure 2. The fifth, other bidentate ligands including 1,10-phenanthroline and diketopyrrolopyrrole (DPP) derivatives could also react with trivalent boron to obtain tetracoordinated organoboron compounds, as shown in Figure 2.
2. Bipyridine-Based Derivatives as Bidentate Ligand
In this section, different traditional methods for the formation of N,N′-chelate organoboron derivatives will be displayed in detail from the following aspects.
2.1. Bipyridine as Bidentate Ligand
In 1985, Heinrich Noeth and co-workers reported that dibutyl(((trifluoromethyl)sulfonyl)oxy)borane (1) reacted with bipyridine (2) to complete desired product. The solution of the diorganylborane should be cooled to −78 °C in this reaction. Bipyridine-dibutylboronium(1+) triflate (3) was confirmed by 11B NMR in their lab [37], as shown in Scheme 1. This protocol realized the synthesis of bipyridine coordinated organoboron complex at low temperature. It played a certain role in promoting the deep study of tetracoordinated organoboron compounds.
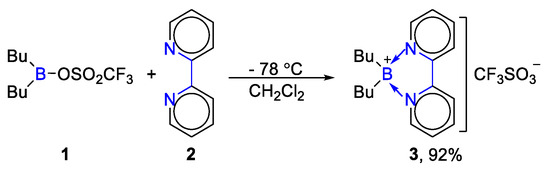
Scheme 1. Reactions of bipyridine with dibutyl(((trifluoromethyl)sulfonyl)oxy)borane [37].
2.2. 2,5-Di(pyridin-2-yl)pyrazine and 2′, 2′: 4′, 4′: 2″, 2‴-Quaterpyridine as Bidentate Ligand
In 2002, Matthias Wagner’s group adopted ferrocenylboranes (4) to react with 2,5-bis(pyridyl)pyrazine (5) and 2′, 2′: 4′, 4′: 2″, 2‴-quaterpyridine (6) to obtain charge-transfer complexes, respectively [38]. The organoboron adducts possess green color, which is charge transfer from the electron-rich ferrocene skeleton to their electron-poor aromatic structures, as shown in Scheme 2. This strategy provided a new route to acquire more ferrocenylborane derivatives with high yield and simple operation.
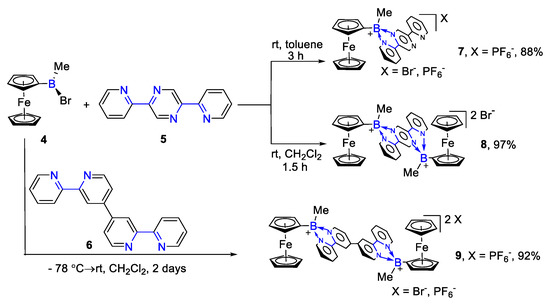
Scheme 2. Reactions of 2,5-di(pyridin-2-yl)pyrazine (or 2′, 2′: 4′, 4′: 2″, 2‴-quaterpyridine) with FcB(Me)Br [38].
2.3. Bipyridine in {Fc(Bbipy)2O}(PF6) and {Fc(Bbipy}2(OH)2}(PF6)
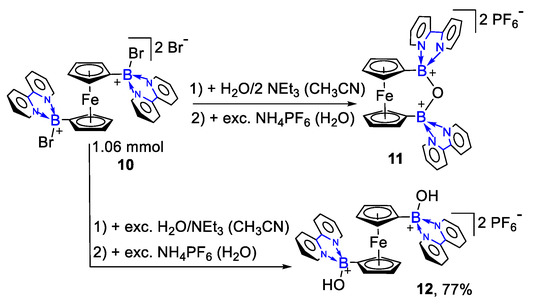
In 2002, Matthias Wagner’s group studied the synthesis of ferrocene complexes {Fc(Bbipy)2O}(PF6)2 (11) and {Fc(Bbipy)2(OH)2}(PF6)2 (12) [39][40], as shown in Scheme 3. They successfully synthesized tetracoordinated organoboron compounds with two boron centers. All of these compounds have the property of charge transfer. They could realize ring-closing and ring-opening products using bromide (FcMeBr) for the formation of 11 and 12 by adding different amounts of water in the reaction.
2.4. 4,4′-Di(but-3-en-1-yl)-2,2′-bipyridine as Bidentate Ligand
In 2005, Matthias Wagner’s team reported other N,N′-chelate organoboron complexes in this reaction. They used dibromo(phenyl)borane, bromo(methyl)(phenyl)borane, bromo(ethoxy)(phenyl)borane to give bipyridine adducts with satisfactory yields (15–18, 76–89%) [41], as shown in Scheme 4. This reaction indicated that different N,N′-chelate organoboron compounds could be obtained by adding distinct boron reagents under ambient temperature.
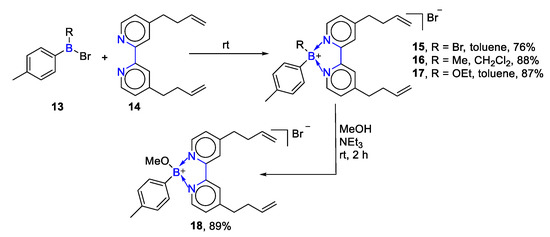
Scheme 4. Reactions of 4,4′-di(but-3-en-1-yl)-2,2′-bipyridine with trivalent boron [41].
2.5. Bipyridine Reacting with 5-Chloro-5,10-dihydrodibenzo[b,e]borinine
In 2009, Warren E. Piers’s lab prepared a series of neutral radicals, which had significant spin density on boron [42]. The scaffold of 2,2′-bipyridyl-stabilized boronium ions was interesting and demonstrated bipyridine adducts persistent neutral radical. They added AgBF4 in this reaction and offered moderate yields (20–23, yield 53–77%), as shown in Scheme 5. It was proved that this protocol could easily get the target 2,2′-bipyridyl boronium ions and neutral radicals.

Scheme 5. Reactions of bipyridine with 5-chloro-5,10-dihydrodibenzo[b,e]borinine [42].
2.6. Bipyridine for the Formation of N,N′-Chelate Organoboron and Ferrocene Derivatives
In 2010, Matthias Wagner’s group reported a more powerful synthetic route of ferrocene complexes [43]. They used bromide (26) to obtain FcBBr polymers (27, 28) and continued to form the bipyridine organoboron polymers with main chain charge-transfer structure (30, 32, 33, 80%, 65%, 76%), as shown in Scheme 6. This method provided a new opportunity for the synthesis of multifunctional organoboron polymers.
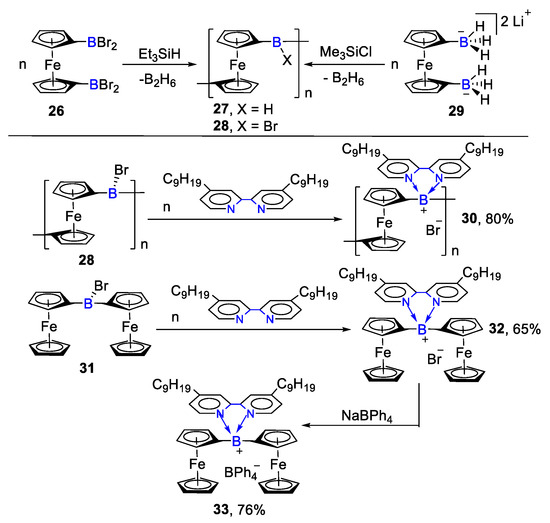
Scheme 6. Bipyridine for the formation of N,N′-chelate organoboron and ferrocene derivatives [43].
2.7. Bipyridine Reacting with 5-Bromo-10-mesityl-5,10-dihydroboranthrene
In 2011, Matthias Wagner’s group has been committed to the development of more diversified N,N′-chelate organoboron chemistry for many years [44]. It always showed wonderful results in bipyridine organoboron adducts. They realized cleavage of the B-O-B bridge in this reaction and got the desired product with moderate yield (36, 51%), as shown in Scheme 7. In this protocol, they prepared 5-bromo-10-mesityl-5,10-dihydroboranthrene (35) as a boron reagent. This building block revealed a novel synthetic route for us.

Scheme 7. Bipyridine reacting with 5-bromo-10-mesityl-5,10-dihydroboranthrene [44].
References
- Maeda, C.; Nagahata, K.; Shirakawa, T.; Ema, T. Azahelicene-Fused BODIPY Analogues Showing Circularly Polarized Lu-minescence. Angew. Chem. Int. Ed. Engl. 2020, 59, 1–6.
- Chi, W.; Chen, J.; Liu, W.; Wang, C.; Qi, Q.; Qiao, Q.; Tan, T.M.; Xiong, K.; Liu, X.; Kang, K.; et al. A General Descriptor DeltaE Enables the Quantitative Development of Luminescent Materials Based on Photoinduced Electron Transfer. J. Am. Chem. Soc. 2020, 142, 6777–6785.
- Gartzia-Rivero, L.; Ray Leiva, C.; Sanchez-Carnerero, E.M.; Banuelos, J.; Moreno, F.; Maroto, B.L.; Garcia-Moreno, I.; Infan-tes, L.; Mendez, B.; Lopez-Arbeloa, I.; et al. Chiral Microneedles from an Achiral Bis(boron dipyrromethene): Spontaneous Mirror Symmetry Breaking Leading to a Promising Photoluminescent Organic Material. Langmuir 2019, 35, 5021–5028.
- Tian, D.; Qi, F.; Ma, H.L.; Wang, X.Q.; Pan, Y.; Chen, R.F.; Shen, Z.; Liu, Z.P.; Huang, L.; Huang, W. Domino-like mul-ti-emissions across red and near infrared from solid-state 2-/2,6-aryl substituted BODIPY dyes. Nat. Commun. 2018, 9, 2688.
- Zhao, Y.; He, S.L.; Yang, J.Y.; Sun, H.; Shen, X.F.; Han, X.G.; Ni, Z.H. Study on TICT emission of TPE-BODIPY derivatives mediated by methyl group on BODIPY. Opt. Mater. 2018, 81, 102–108.
- Li, G.; Hirano, T.; Yamada, K. Bright near-infrared chemiluminescent dyes: Phthalhydrazides conjugated with fluorescent BODIPYs. Dyes Pigm. 2020, 178, 108339.
- Radunz, S.; Kraus, W.; Bischoff, F.A.; Emmerling, F.; Tschiche, H.R.; Resch-Genger, U. Temperature- and Struc-ture-Dependent Optical Properties and Photophysics of BODIPY Dyes. J. Phys. Chem. A. 2020, 124, 1787–1797.
- Boens, N.; Verbelen, B.; Ortiz, M.J.; Jiao, L.; Dehaen, W. Synthesis of BODIPY dyes through postfunctionalization of the bo-ron dipyrromethene core. Coord. Chem. Rev. 2019, 399, 213024.
- Li, G.L.; Otsuka, Y.; Matsumiya, T.; Suzuki, T.; Li, J.Y.; Takahashi, M.; Yamada, K. A Straightforward Substitution Strategy to Tune BODIPY Dyes Spanning the Near-Infrared Region via Suzuki-Miyaura Cross-Coupling. Materials 2018, 11, 1297.
- Cao, J.J.; Zhang, M.S.; Li, X.Q.; Yang, D.C.; Xu, G.; Liu, J.Y. A glutathione-responsive photosensitizer with fluorescence reso-nance energy transfer characteristics for imaging-guided targeting photodynamic therapy. Eur. J. Med. Chem. 2020, 193, 112203.
- Lapok, L.; Cieslar, I.; Pedzinski, T.; Stadnicka, K.M.; Nowakowska, M. Near-Infrared Photoactive Aza-BODIPY: Thermally Robust and Photostable Photosensitizer and Efficient Electron Donor. Chemphyschem 2020, 21, 1–7.
- Wang, C.J.; Qian, Y. A water soluble carbazolyl-BODIPY photosensitizer with orthogonal D-A structure for photodynamic therapy in living cells and zebrafish. Biomater. Sci. 2020, 8, 830–836.
- Wang, C.J.; Qian, Y. A novel BODIPY-based photosensitizer with pH-active singlet oxygen generation for photodynamic therapy in lysosomes. Org. Biomol. Chem. 2019, 17, 8001–8007.
- Wang, L.F.; Bai, J.; Qian, Y. Synthesis of a triphenylamine BODIPY photosensitizer with D–A configuration and its applica-tion in intracellular simulated photodynamic therapy. New J. Chem. 2019, 43, 16829–16834.
- Dolati, H.; Haufe, L.C.; Denker, L.; Lorbach, A.; Grotjahn, R.; Horner, G.; Frank, R. Two pi-Electrons Make the Difference: From BODIPY to BODIIM Switchable Fluorescent Dyes. Chem. Eur. J. 2020, 26, 1422–1428.
- Gautam, R.; Petritis, S.J.; Tomat, E. Redox-Switchable Cyan Fluorescence of a BODIPY Analog Inspired by Propentdyopent Pigments. Eur. J. Inorg. Chem. 2019, 2019, 68–72.
- Shi, W.-J.; Huang, Y.; Liu, W.C.; Xu, D.; Chen, S.-T.; Liu, F.G.; Hu, J.Q.; Zheng, L.Y.; Chen, K. A BODIPY-based “OFF-ON” fluorescent probe for fast and selective detection of hypochlorite in living cells. Dyes Pigm. 2019, 170, 107566.
- Nguyen, V.N.; Yim, Y.; Kim, S.; Ryu, B.; Swamy, K.M.K.; Kim, G.; Kwon, N.; Kim, C.Y.; Park, S.; Yoon, J. Molecular Design of Highly Efficient Heavy-Atom-Free Triplet BODIPY Derivatives for Photodynamic Therapy and Bioimaging. Angew. Chem. Int. Ed. Engl. 2020, 59, 1–7.
- Pederzoli, M.; Wasif Baig, M.; Kyvala, M.; Pittner, J.; Cwiklik, L. Photophysics of BODIPY-Based Photosensitizer for Photo-dynamic Therapy: Surface Hopping and Classical Molecular Dynamics. J. Chem. Theory. Comput. 2019, 15, 5046–5057.
- Gayathri, T.; Vijayalakshmi, A.; Mangalath, S.; Joseph, J.; Rao, N.M.; Singh, S.P. Study on Liposomal Encapsulation of New Bodipy Sensitizers for Photodynamic Therapy. ACS. Med. Chem. Lett. 2018, 9, 323–327.
- Dai, X.M.; Chen, X.L.; Zhao, Y.; Yu, Y.J.; Wei, X.S.; Zhang, X.G.; Li, C.X. A Water-Soluble Galactose-Decorated Cationic Photodynamic Therapy Agent Based on BODIPY to Selectively Eliminate Biofilm. Biomacromolecules 2018, 19, 141–149.
- Huang, L.; Li, Z.J.; Zhao, Y.; Zhang, Y.W.; Wu, S.; Zhao, J.Z.; Han, G. Ultralow-Power Near Infrared Lamp Light Operable Targeted Organic Nanoparticle Photodynamic Therapy. J. Am. Chem. Soc. 2016, 138, 14586–14591.
- Wu, S.; Li, Y.; Deng, T.; Wang, X.; Hu, S.; Peng, G.; Huang, X.A.; Ling, Y.; Liu, F. A new fluorescent probe for sensing of bio-thiols and screening of acetylcholinesterase inhibitors. Org. Biomol. Chem. 2020, 18, 2468–2474.
- Zhang, J.; Wang, N.; Ji, X.; Tao, Y.; Wang, J.; Zhao, W. BODIPY-Based Fluorescent Probes for Biothiols. Chem. Eur. J. 2020, 26, 4172–4192.
- Guria, S.; Ghosh, A.; Manna, K.; Pal, A.; Adhikary, A.; Adhikari, S. Rapid detection of aspartic acid and glutamic acid in water by BODIPY-Based fluorescent probe: Live-cell imaging and DFT studies. Dyes Pigm. 2019, 168, 111–122.
- Wijesooriya, C.S.; Peterson, J.A.; Shrestha, P.; Gehrmann, E.J.; Winter, A.H.; Smith, E.A. A Photoactivatable BODIPY Probe for Localization-Based Super-Resolution Cellular Imaging. Angew. Chem. Int. Ed. Engl. 2018, 57, 12685–12689.
- Brommel, K.; Maskri, S.; Maisuls, I.; Konken, C.P.; Rieke, M.; Petho, Z.; Strassert, C.A.; Koch, O.; Schwab, A.; Wunsch, B. Synthesis of Small-Molecule Fluorescent Probes for the In Vitro Imaging of Calcium-Activated Potassium Channel KCa3.1. Angew. Chem. Int. Ed. Engl. 2020, 59, 2–10.
- Taniguchi, A.; Sawazaki, T.; Shimizu, Y.; Sohma, Y.; Kanai, M. Photophysical properties and application in live cell imaging of B,B-fluoro-perfluoroalkyl BODIPYs. Med. Chem. Commun. 2019, 10, 1121–1125.
- Zhang, P.-L.; Wang, Z.-K.; Chen, Q.-Y.; Du, X.; Gao, J. Biocompatible G-Quadruplex/BODIPY assembly for cancer cell imag-ing and the attenuation of mitochondria. Bioorg. Med. Chem. Lett. 2019, 29, 1943–1947.
- Liu, Q.D.; Mudadu, M.S.; Thummel, R.; Tao, Y.; Wang, S. From Blue to Red: Syntheses, Structures, Electronic and Electrolu-minescent Properties of Tunable Luminescent N,N Chelate Boron Complexes. Adv. Funct. Mater. 2005, 15, 143–154.
- Yang, T.; Cao, X.; Zhang, X.X.; Ou, Y.; Au, C.T.; Yin, S.F.; Qiu, R. Iodine-Catalyzed Synthesis of N,N-Chelate Organoboron Aminoquinolate. J. Org. Chem. 2020, 85, 12430–12443.
- Mas-Montoya, M.; Usea, L.; Espinosa Ferao, A.; Montenegro, M.F.; Ramirez de Arellano, C.; Tarraga, A.; Rodriguez-Lopez, J.N.; Curiel, D. Single Heteroatom Fine-Tuning of the Emissive Properties in Organoboron Complexes with 7-(Azaheteroaryl)indole Systems. J. Org. Chem. 2016, 81, 3296–3302.
- Shiu, Y.J.; Cheng, Y.C.; Tsai, W.L.; Wu, C.C.; Chao, C.T.; Lu, C.W.; Chi, Y.; Chen, Y.T.; Liu, S.H.; Chou, P.T. Pyridyl Pyrrol-ide Boron Complexes: The Facile Generation of Thermally Activated Delayed Fluorescence and Preparation of Organic Light-Emitting Diodes. Angew. Chem. Int. Ed. Engl. 2016, 55, 3017–3021.
- Nagata, Y.; Chujo, Y. Main-Chain-Type N,N'-Chelate Organoboron Aminoquinolate Polymers: Synthesis, Luminescence, and Energy Transfer Behavior. Macromolecules 2008, 41, 3488–3492.
- Zu, W.; Day, C.; Wei, L.; Jia, X.; Xu, L. Dual aminoquinolate diarylboron and nickel catalysed metallaphotoredox platform for carbon-oxygen bond construction. Chem. Commun. 2020, 56, 8273–8276.
- Liu, S.-F.; Wu, Q.; Schmider, H.L.; Aziz, H.; Hu, N.-X.; Popovic´, Z.; Wang, S. Syntheses, Structures, and Electrolumines-cence of New Blue/Green Luminescent Chelate Compounds: Zn(2-py-in)2(THF), BPh2(2-py-in), Be(2-py-in)2, and BPh2(2-py-aza) [2-py-in ) 2-(2-pyridyl)indole; 2-py-aza ) 2-(2-pyridyl)-7-azaindole]. J. Am. Chem. Soc. 2000, 122, 3671–3678.
- Narula, C.K.; Noeth, H. Competition between adduct and cation formation in reactions between diorganylborane derivatives and pyridine or lutidines. Inorg. Chem. 1985, 24, 2532–2539.
- Ding, L.; Ma, K.; Dürner, G.; Bolte, M.; Fabrizi de Biani, F.; Zanello, P.; Wagner, M. Reactions of ferrocenylboranes with 2,5-bis(pyridyl)pyrazine and quaterpyridine: Charge-transfer complexes and redox-active macrocycles. J. Chem. Soc., Dalton Trans. 2002, 8, 1566–1573.
- Ding, L.; Ma, K.; Fabrizi de Biani, F.; Bolte, M.; Zanello, P.; Wagner, M. Electronic Communication in a Novel Five-Step Re-dox System. Organometallics 2001, 20, 1041–1043.
- Ma, K.; Fabrizi de Biani, F.; Bolte, M.; Zanello, P.; Wagner, M. Electronic Communication in a Series of Novel Multistep Re-dox Systems. Organometallics 2002, 21, 3979–3989.
- Haberecht, M.C.; Bolte, M.; Lerner, H.-W.; Wagner, M. An Approach Towards Functional Dendrimers Based on the Hy-droboration Reaction and Spontaneous Boron-Nitrogen Bond Formation. Eur. J. Inorg. Chem. 2005, 2005, 4309–4316.
- Wood, T.K.; Piers, W.E.; Keay, B.A.; Parvez, M. Spirocyclic boronium ions: Precursors to persistent neutral radicals. Chem. Commun. 2009, 34, 5147–5149.
- Cui, C.; Heilmann-Brohl, J.; Sánchez Perucha, A.; Thomson, M.D.; Roskos, H.G.; Wagner, M.; Jäkle, F. Redox-Active Ferro-cenylboronium Polyelectrolytes with Main Chain Charge-Transfer Structure. Macromolecules 2010, 43, 5256–5261.
- Januszewski, E.; Lorbach, A.; Grewal, R.; Bolte, M.; Bats, J.W.; Lerner, H.W.; Wagner, M. Unsymmetrically substituted 9,10-dihydro-9,10-diboraanthracenes as versatile building blocks for boron-doped pi-conjugated systems. Chem. Eur. J. 2011, 17, 12696–12705.