Very long-chain (˃C24) fatty acids (VLC-FA) play critical roles during early development of vertebrates, since these compounds are accumulated in the rapidly forming neural tissues, ensuring their normal function. However, despite their putative importance, the study of VLC-FA in fish is scarce. Biosynthesis of VLC-FA is carried out by the so-called elongation of very long-chain fatty acid 4 (Elovl4) proteins and, consequently, the complement and function of these enzymes determine the capacity that a given species has for satisfying the physiological demands for VLC-FA, especially during its early development. The present study aimed to characterize elovl4 genes from the marine teleosts Sparus aurata and Solea senegalensis, and determine the function of the corresponding encoded proteins. Moreover, the tissue expression pattern of elovl4 genes was determined. The results confirmed that both fish species possess two distinct Elovl4 proteins termed as Elovl4a and Elovl4b based on their homology to the zebrafish orthologs. Functional assays in yeast denoted that both Elovl4a and Elovl4b from both species had the capability to elongate C20-24 fatty acid precursors to VLC-FA products. However, Elovl4b appeared to have a higher activity than Elovl4a elongating all the polyunsaturated fatty acid substrates assayed to longer chain polyunsaturated products, especially on the n-3 series. Gene expression results indicated that, although elovl4 transcripts were detected in most tissues analyzed, elovl4 genes were more strongly expressed in both species neural tissues such as brain and eyes, which showed the highest expression levels of elovl4a and elovl4b, respectively. These results are consistent with the functions of Elovl4 from other vertebrates. Importantly, these findings contribute to a better understanding of the VLC-FA biosynthetic pathway in marine teleosts, highlighting the crucial role that Elovl4 products carry out for the correct development and maintenance of neurophysiologic functions during early stages of the fish development.
- very long-chain polyunsaturated fatty acid
- Elovl4
- fish
1. Introduction
Certain long-chain (C
20-24
) polyunsaturated fatty acids (LC-PUFA), namely eicosapentaenoic acid (EPA; 20:5n-3), arachidonic acid (ARA; 20:4n-6) and docosahexaenoic acid (DHA; 22:6n-3), are regarded as physiologically essential for the correct development of vertebrates, including fish [1]. These compounds can be obtained through the diet or, alternatively, biosynthesized from C
18 polyunsaturated fatty acids (PUFA), such as α-linolenic acid (18:3n-3) and linoleic acid (18:2n-6) via enzymatic reactions carried out by fatty acyl desaturases (Fads) and elongation of very long-chain fatty acid (Elovl) proteins [1-2]. Fads are enzymes that introduce double bonds (unsaturations) into PUFA substrates. On the other hand, Elovl are considered pivotal components of fatty acid (FA) synthetic pathways [3-4], being responsible for a condensation reaction which results in the extension of the pre-existing FA chain with two new carbon atoms [1]. The Elovl protein family contains several members (Elovl1-8) [2-6], but only Elovl4 catalyzes the synthesis of very long-chain (>C
polyunsaturated fatty acids (PUFA), such as α-linolenic acid (18:3n-3) and linoleic acid (18:2n-6) via enzymatic reactions carried out by fatty acyl desaturases (Fads) and elongation of very long-chain fatty acid (Elovl) proteins [1][2]. Fads are enzymes that introduce double bonds (unsaturations) into PUFA substrates. On the other hand, Elovl are considered pivotal components of fatty acid (FA) synthetic pathways [3][4], being responsible for a condensation reaction which results in the extension of the pre-existing FA chain with two new carbon atoms [1]. The Elovl protein family contains several members (Elovl1-8) [2][3][4][5][6], but only Elovl4 catalyzes the synthesis of very long-chain (>C
24) saturated (VLC-SFA) and polyunsaturated fatty acids (VLC-PUFA), which can have up to 36 or 38 carbons [1,7].
) saturated (VLC-SFA) and polyunsaturated fatty acids (VLC-PUFA), which can have up to 36 or 38 carbons [1][7]. Furthermore, Elovl4 is additionally responsible for the production of very long-chain saturated fatty acids (VLC-SFA) [8].
Virtually all teleosts possess at least two Elovl4 isoforms termed as “Elovl4a” and “Elovl4b” [2,9]. However, their functions seem to vary among species, highlighting that the investigation of Elovl4 proteins in teleosts requires a species-specific approach. The gilthead seabream (
Virtually all teleosts possess at least two Elovl4 isoforms termed as “Elovl4a” and “Elovl4b” [2][9]. However, their functions seem to vary among species, highlighting that the investigation of Elovl4 proteins in teleosts requires a species-specific approach. The gilthead seabream (
Sparus aurata
) and Senegalese sole (
Solea senegalensis) are two commercially important species in marine finfish aquaculture. A recent study has highlighed a relationship between the expression of
) are two commercially important species in marine finfish aquaculture. A recent study has highlighed a relationship between the expression of
elovl4 genes in both species and the formation of neural tissues during early life-cycle development [12]. Indeed, Elovl4 products, i.e. VLC-SFA and VLC-PUFA, play crucial roles during early-development of vertebrates by guaranteeing the correct development and functionality of the rapidly forming nervous system where these compounds accumulate [8,12]. Thus, due to the importance of very long-chain fatty acids (VLC-FA) during early development, it is crucial to understand the capacity that a given species has for endogenous production of these essential nutrients. Such ability is itself dependent on the complement of
genes in both species and the formation of neural tissues during early life-cycle development [10]. Indeed, Elovl4 products, i.e. VLC-SFA and VLC-PUFA, play crucial roles during early-development of vertebrates by guaranteeing the correct development and functionality of the rapidly forming nervous system where these compounds accumulate [8][10]. Thus, due to the importance of very long-chain fatty acids (VLC-FA) during early development, it is crucial to understand the capacity that a given species has for endogenous production of these essential nutrients. Such ability is itself dependent on the complement of
elovl4 genes and the functions of their corresponding encoded enzymes [1]. Previous studies investigating the functions of
genes and the functions of their corresponding encoded enzymes [1]. Previous studies investigating the functions of
fads- and other
- and other
elovl-like genes confirmed that both fish species operate different LC-PUFA biosynthesis mechanisms [13-16], especially with regard to production of DHA. In particular,
-like genes confirmed that both fish species operate different LC-PUFA biosynthesis mechanisms [11][12][13][14], especially with regard to production of DHA. In particular,
Sa operates the so-called “Sprecher pathway” [13, 17] whereas
operates the so-called “Sprecher pathway” [11][15] whereas
Ss produces DHA via the more direct “Δ4 pathway” [16] (
produces DHA via the more direct “Δ4 pathway” [14] (
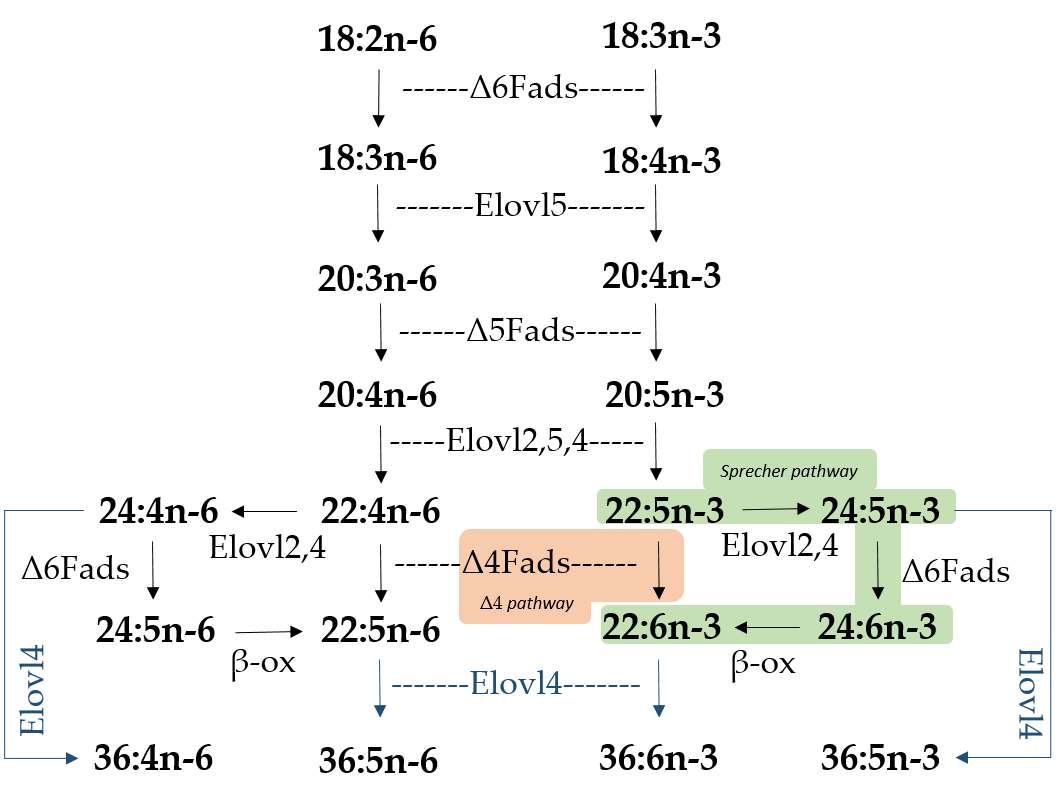
Figure 1). Considering that both the LC-PUFA and VLC-PUFA biosynthetic pathways are interdependent, the aim of the present study was to characterize, both molecularly and functionally,
). Considering that both the LC-PUFA and VLC-PUFA biosynthetic pathways are interdependent, the aim of the present study was to characterize, both molecularly and functionally,
elovl4 paralogs from
paralogs from
S. aurata (
(
Sa) and
) and
S. senegalensis (
(
Ss) and discuss the biosynthetic particularities of both species.
) and discuss the biosynthetic particularities of both species.
Figure 1.
Biosynthetic pathways of long-chain (C
20-24
) and very long-chain polyunsaturated fatty acids (VLC-PUFA; >C
24
) in fish. Desaturation reactions are mediated by fatty acyl desaturases (Fads), whereas elongation reactions are catalyzed by elongation of very long-chain fatty acid (Elovl) proteins. Microsomal β-oxidation reactions are denoted as “β-ox”. Two pathways for docosahexaenoic acid (22:6n-3) biosynthesis from docosapentaenoic acid (22:5n-3) are indicated, namely the Sprecher pathway (green background) and the Δ4 pathway (orange background). Elongation reactions leading to VLC-PUFA biosynthesis of up to C
36
are indicated with blue arrows. Note the fish species studied herein (
Sparus aurata
and
Solea senegalensis
) lack
elovl2
in their genomes [2].
Materials and Methods
2. Materials and Methods
2.1. Molecular cloning of elovl4 cDNA sequences
Cloning of the
elovl4
full-length cDNA was carried out using PCR-based methodologies and brain-eye mix (1:1) cDNA as template. The PCR fragments were then purified and sequenced at least two times. In order to obtain the full-length ORF sequences, two-round (nested) Rapid Amplification of cDNA ends (RACE) PCR were performed. Potential positive fragments were cloned into pGEM-T Easy cloning vector and ligated with T4 DNA ligase. The plasmid preparations were purified and sequenced. The putative
elovl4
sequences were thus obtained and deposited in the GenBank database as gb|MK610320 (
Sa
elovl4a
), gb|MK610321 (
Sa
elovl4b
), gb|MN164537 (
elovl4a
) and gb|MN164625 (
elovl4b
).
Sequence and phylogenetic analysis
2.2. Sequence and phylogenetic analysis
The BLAST sequence analysis of NCBI was used for sequence alignment and phylogenetic analysis. The aa sequences deduced from the nucleotide sequences of the
Sa
Elovl4a (gb|QES86604.1),
Ss
Elovl4a (gb|QGA31141.1),
Sa
Elovl4b (gb|QES86605.1), and
Ss
Elovl4b (gb|QGA31140.1), were aligned using the ClustalW tool (BioEdit v7.0.9). The phylogenetic tree was constructed using a total of 31 Elovl aa sequences from different species using MEGA X software.
2.3. Functional characterization of Sa and Ss Elovl4 isoforms
The
Sa
and
Ss
putative Elovl4 elongases were functionally characterized by determining the FA profiles of
Saccharomyces cerevisiae
transformed with pYES2 yeast expression vector containing the putative
elovl4
as inserts, and grown in the presence of potential FA substrates.
Fatty acid analysis
2.4. Fatty acid analysis
The elongation of endogenous saturated FA with 24 carbons or longer was assessed by comparison of the areas of the fatty acids of control yeast with those of yeast transformed with each of the pYES2-elovl4 plasmid constructs (n=3). In the case of VLC-PUFA, the elongation conversions of exogenously added PUFA were calculated as (area of first product and longer chain products/(area of first product and longer chain products + substrate area)) x 100.
The elongation of endogenous saturated FA with 24 carbons or longer was assessed by comparison of the areas of the fatty acids of control yeast with those of yeast transformed with each of the pYES2-elovl4 plasmid constructs (n=3). In the case of VLC-PUFA, the elongation conversions of exogenously added PUFA were calculated as (area of first product and longer chain products/(area of first product and longer chain products + substrate area)) × 100.
2.5. Tissue expression of elovl4 genes in gilthead seabream and Senegalese sole
Expression of elovl4 isoforms in each tissue from one specimen of gilthead seabream and Senegalese sole was analyzed by reverse transcriptase PCR (RT-PCR), using 18s ribosomal RNA (18s) as a reference gene. Expression of elovl4a and elovl4b in selected tissues that showed a strong signal in RT-PCR analyses (brain, eye and gonad) was analyzed by qPCR using β-actin (actb) as reference gene (n=3).
Statistical analysis
2.6. Statistical analysis
The homogeneity of variances of the data associated to VLC-SFA (%) and tissue gene expression values, determined by qPCR, were checked using Levene’s test. Statistical differences were analyzed by one-way analysis of variance (ANOVA) (
P
≤ 0.05) followed by Tukey HSD
post-hoc
tests using SPSS 26.0 software.
Results
3. Results
Elovl4 sequence and phylogenetic analysis
3.1. Elovl4 sequence and phylogenetic analysis
The
Sa
and
Ss elovl4a
ORF sequences have 969 base pairs (bp) and 960 bp, encoding putative proteins of 322 amino acids (aa) and 319 aa, respectively. On the other hand,
Sa
and
Ss
elovl4b
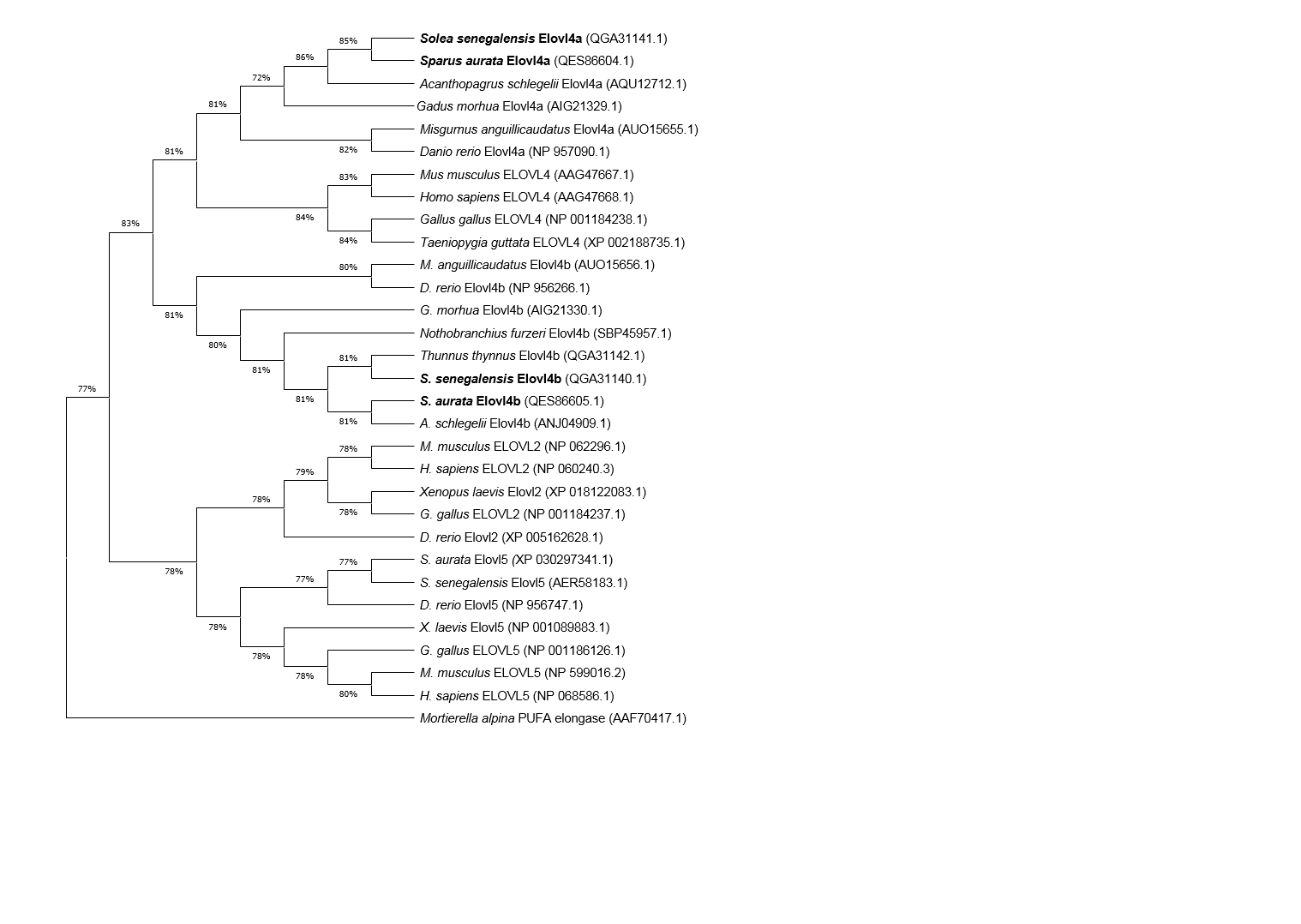
ORF sequences contain 918 bp, encoding proteins of 305 aa. The phylogenetic analysis showed that both Elovl4 sequences from each species form two separate clusters that include either Elovl4a or Elovl4b sequences from a range of teleosts (Figure 2).
Figure 2.
Phylogenetic tree comparing
Sparus aurata
and
Solea senegalensis
Elovl4a and Elovl4b proteins (highlighted in bold) with Elovl2, Elovl4 and Elovl5 proteins from other vertebrates. The tree was constructed using the Maximum Likelihood method and JTT matrix-based model. The numbers in branches represent the frequencies (%) of each node after 1000 iterations by bootstrapping. The
Mortierella alpina
PUFA elongase was included in the analysis as an outgroup, to construct the rooted tree.
Functional characterization of Elovl4a and Elovl4b
3.2. Functional characterization of Elovl4a and Elovl4b
The results confirmed that both
Sa
and
Ss
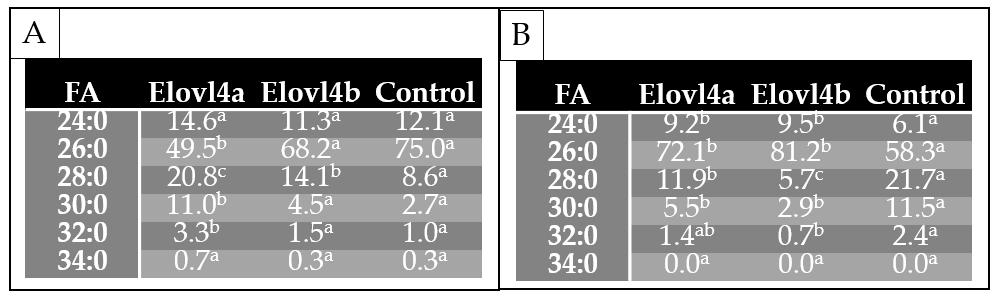
Elovl4 isoforms are involved in the biosynthesis of VLC-SFA (
Table 1A-B
). These results suggest that 26:0 is an important substrate for both
Sa
Elovl4 proteins (
Table 1A
). Moreover,
Ss
Elovl4 proteins are involved in the biosynthesis of VLC-SFA up to 26:0 for which they use <C
24
fatty acids as elongation substrates (
Table 1B
). Furthermore, the chromatographic analyses of Elovl4-transformed yeast revealed that
Sa
Elovl4a elongated all n-6 PUFA substrates, as well as n-3 PUFA substrates (
Table 2
).
Sa
Elovl4b also elongated n-6 PUFA substrates, but showed particularly high affinity towards n-3 PUFA substrates, especially to elongate 22:6n-3 (DHA) substrates to 32:6n-3 (
Table 2
). For Senegalese sole, both Elovl4 elongases presented the capability to elongate PUFA substrates from the n-3 and n-6 series, to longer chain FA of up to C
34
(
Table 2
). It is noteworthy that
Ss
Elovl4b, and to a lesser extent Elovl4a, showed high capacity to elongate 20:5n-3 (EPA) to 22:5n-3, a key step required for DHA synthesis via the Δ4 pathway.
Table 1. Functional characterization of Sparus aurata (A) and Solea senegalensis (B) Elovl4 elongases: role in the biosynthesis of very long-chain saturated fatty acids (FA). Results are expressed as area percentage (%) of total saturated FA ≥ C24 found in yeast transformed with either pYES2 containing the elovl4 coding regions or empty pYES2 vector (control) (n=3). Different superscripts denote significant differences in each row, among area percentages of each saturated FA (one way-ANOVA and Tukey test, P ≤ 0.05).
|
|
Sa Elovl4a |
Sa Elovl4b |
Ss Elovl4a |
Ss Elovl4b |
|
|
FA substrate |
Product |
% Conversion |
|||
|
18:4n-3 |
20:4n-3 |
2.5 |
2.7 |
4.5 |
8.1 |
|
22:4n-3 |
9.7 |
12.5 |
19.6 |
41.2 |
|
|
24:4n-3 |
5.6 |
49.9 |
39.5 |
79.0 |
|
|
26:4n-3 |
n.d. |
65.6 |
39.6 |
95.3 |
|
|
28:4n-3 |
n.d. |
n.d. |
100 |
96.8 |
|
|
30:4n-3 |
n.d. |
n.d. |
100 |
98.7 |
|
|
32:4n-3 |
n.d. |
n.d. |
65.4 |
65.7 |
|
|
34:4n-3 |
n.d. |
n.d. |
n.d. |
1.7 |
|
|
18:3n-6 |
20:3n-6 |
2.6 |
2.1 |
4.6 |
6.2 |
|
22:3n-6 |
21.6 |
9.6 |
38.6 |
40.8 |
|
|
24:3n-6 |
52.5 |
n.d. |
66.2 |
66.0 |
|
|
26:3n-6 |
57.1 |
n.d. |
65.3 |
89.1 |
|
|
28:3n-6 |
64.8 |
n.d. |
100 |
91.9 |
|
|
30:3n-6 |
90.0 |
n.d. |
55.0 |
90.4 |
|
|
32:3n-6 |
84.1 |
n.d. |
62.7 |
17.8 |
|
|
34:3n-6 |
41.3 |
n.d. |
n.d. |
n.d. |
|
|
20:5n-3 |
22:5n-3 |
5.8 |
9.1 |
12.1 |
30.9 |
|
24:5n-3 |
17.2 |
33.3 |
31.8 |
75.1 |
|
|
26:5n-3 |
20.0 |
57.8 |
35.7 |
87.4 |
|
|
28:5n-3 |
n.d. |
86.8 |
100 |
96.9 |
|
|
30:5n-3 |
n.d. |
97.7 |
50.0 |
98.9 |
|
|
32:5n-3 |
n.d. |
72.7 |
33.7 |
82.9 |
|
|
34:5n-3 |
n.d. |
8.1 |
38.2 |
14.5 |
|
|
20:4n-6
|
22:4n-6 |
10.9 |
8.9 |
18.1 |
33.1 |
|
24:4n-6 |
31.0 |
30.2 |
49.9 |
73.4 |
|
|
26:4n-6 |
37.1 |
55.9 |
56.7 |
85.1 |
|
|
28:4n-6 |
39.0 |
81.0 |
65.2 |
94.3 |
|
|
30:4n-6 |
88.6 |
37.8 |
95.2 |
95.9 |
|
|
32:4n-6 |
83.6 |
n.d. |
84.9 |
51.8 |
|
|
34:4n-6 |
73.7 |
n.d. |
25.3 |
2.7 |
|
|
36:4n-6 |
11.4 |
n.d. |
n.d. |
n.d. |
|
|
22:5n-3 |
24:5n-3 |
3.4 |
12.6 |
7.8 |
44.3 |
|
26:5n-3 |
19.8 |
52.2 |
33.9 |
87.9 |
|
|
28:5n-3 |
26.0 |
86.3 |
51.2 |
97.0 |
|
|
30:5n-3 |
85.6 |
96.5 |
92.3 |
99.0 |
|
|
32:5n-3 |
74.2 |
64.4 |
27.4 |
82.5 |
|
|
34:5n-3 |
63.0 |
5.3 |
32.4 |
16.2 |
|
|
22:4n-6
|
24:4n-6 |
8.2 |
10.4 |
13.5 |
37.2 |
|
26:4n-6 |
35.1 |
43.1 |
58.3 |
85.5 |
|
|
28:4n-6 |
45.5 |
71.8 |
71.8 |
94.5 |
|
|
30:4n-6 |
90.8 |
83.0 |
94.5 |
96.3 |
|
|
32:4n-6 |
78.7 |
19.5 |
21.6 |
53.9 |
|
|
34:4n-6 |
54.6 |
n.d. |
25.9 |
5.0 |
|
|
36:4n-6 |
7.2 |
n.d. |
n.d. |
n.d. |
|
|
22:6n-3 |
24:6n-3 |
0.4 |
1.8 |
0.6 |
5.1 |
|
26:6n-3 |
n.d. |
100 |
n.d. |
100 |
|
|
28:6n-3 |
n.d. |
100 |
n.d. |
100 |
|
|
30:6n-3 |
n.d. |
40.2 |
n.d. |
100 |
|
|
32:6n-3 |
n.d. |
61.3 |
n.d. |
22.3 |
|
|
34:6n-3 |
n.d. |
n.d. |
n.d. |
n.d. |
|
n.d.: not detected.
Table 2. Functional characterization of the Sparus aurata (Sa) and Solea senegalensis (Ss) Elovl4a and Elovl4b elongases by heterologous expression in the yeast Saccharomyces cerevisiae. Data are presented as the percentage conversions of polyunsaturated fatty acid (FA) substrates (n=1). Individual conversions were calculated according to the formula (area of first product and longer chain products/(area of first product and longer chain products + substrate area)) x 100. Functional characterization of the Sparus aurata (Sa) and Solea senegalensis (Ss) Elovl4a and Elovl4b elongases by heterologous expression in the yeast Saccharomyces cerevisiae. Data are presented as the percentage conversions of polyunsaturated fatty acid (FA) substrates (n=1). Individual conversions were calculated according to the formula (area of first product and longer chain products/(area of first product and longer chain products + substrate area)) × 100.
Tissue expression of
3.3. Tissue expression of
elovl4
genes
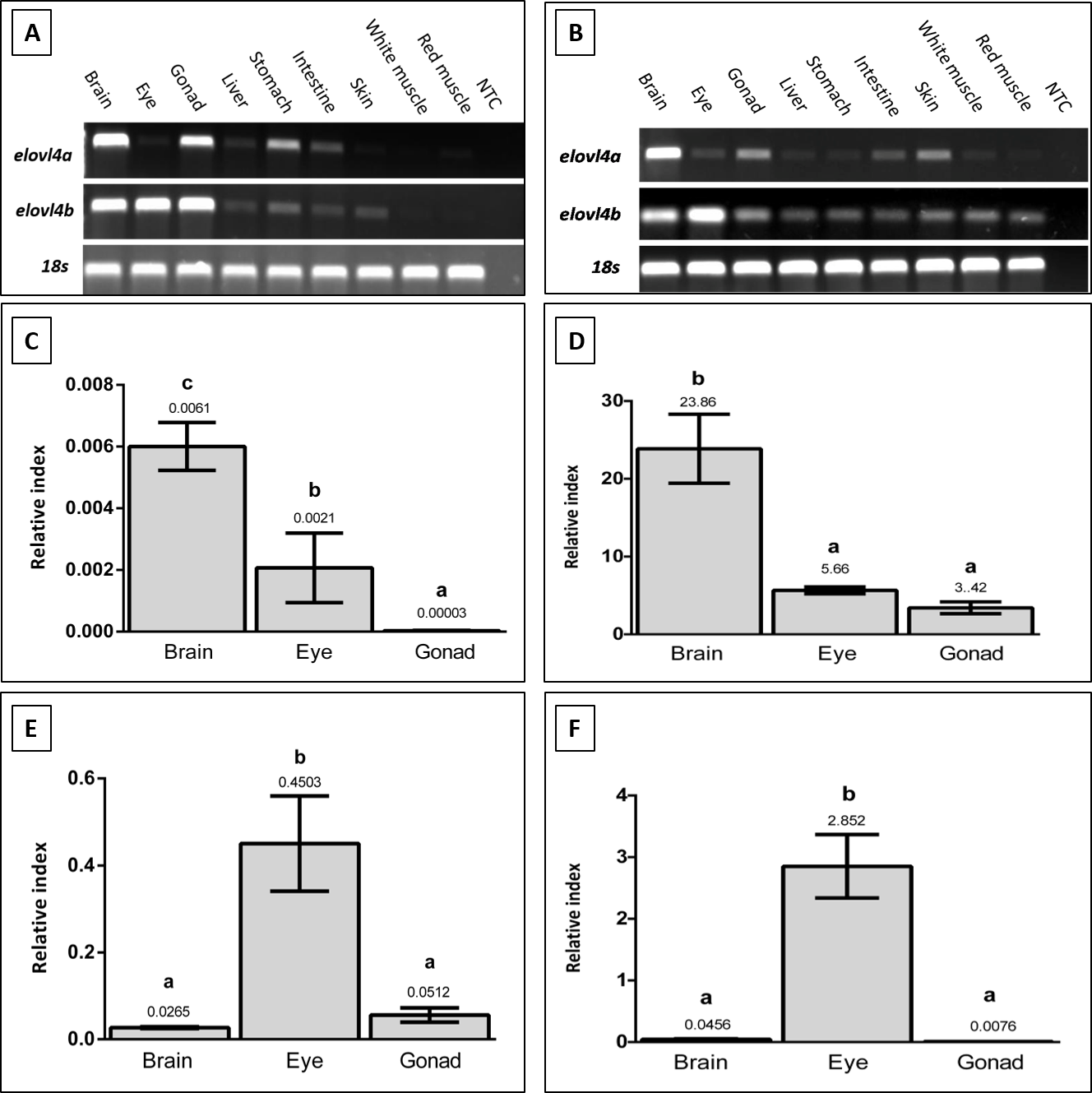
RT-PCR analyses denoted that, in both species,
elovl4a
and
elovl4b
transcripts appear to be present in most of the analyzed tissues (
Figure 3A-B
). RT-PCR analyses indicated that for both species, highest expression values were found in brain for
elovl4a
, and eye for
elovl4b
(
Figure 3C-F
).
Figure 3. Tissue distribution of elovl4a and elovl4b transcripts in Sparus aurata (A) and Solea senegalensis (B) determined by RT-PCR (n=1 fish). Expression of housekeeping gene 18s is also shown. Expression in selected tissues of Sparus aurata elovl4a (C) Solea senegalensis elovl4a (D), Sparus aurata elovl4b (E) and Solea senegalensis elovl4b (F) transcripts was also determined by qPCR. The results, shown as relative index, are β-actin normalized values (gene copy number/β-actin copy number). Bars represent means and standard deviations (n=3 fish). Different letters denote significant differences (ANOVA and Tukey HSD test, P ≤ 0.05) among tissues.
Discussion
4. Discussion
Sequence analyses revealed that the investigated predicted Elovl4 proteins contain all characteristic domains of vertebrate Elovl4 family members [18-19][16][17]. Moreover, phylogenetic analysis confirmed that the described Elovl4 isoforms are true orthologs of the Elovl4a and Elovl4b proteins present in teleosts [2]. So, the conservation of both Elovl4 isoforms in fish genomes [2,9-11,20,21][2][9][18][19][20][21] and their clear segregation into separate clusters points towards a likely functional specialization of these proteins in teleosts [22[22][23],23], which we aimed to further elucidate in this study by functionally characterizing the two isoforms in two new fish species with diverse life histories, dietary habits, and notably different LC-PUFA biosynthesis mechanisms [13-16][11][12][13][14].
As suggested in previous studies with other fish species, including Danio rerio [9], Clarias gariepinus [10] [18] and Salmo salar [24], our results support the notion that both isoforms can participate in VLC-SFA elongation. Nevertheless, similarly to what was reported in D. rerio [9], Elovl4a seems to be more efficient than Elovl4b at elongating VLC-SFA. Notably, the functional characterization of Ss Elovl4 enzymes showed some differences with respect to Sa, particularly concerning the preferred fatty acid substrates. Moreover, functional analyses of Elovl4a and Elovl4b confirmed that both proteins participate actively in the biosynthesis of either n-6 or n-3 VLC-PUFA, from n-6 and n-3 PUFA substrates, in the two studied fish species. However, intra- and inter-specific differences were found in the efficiency of the different Elovl4 isoforms to biosynthesize VLC-PUFA. This differences could be related with different VLC-SFA and VLC-PUFA requirements between the two fish species [12][10], but further studies are necessary to clearly establish this. It is also noteworthy that, similar to what has been described in zebrafish [9], both Sa and Ss Elovl4a elongases showed low elongation activity from DHA to 24:6n-3. This could suggest that, as described in rat retinas [25], EPA and not DHA might be the preferred substrate for VLC-PUFA biosynthesis in fish. Similarly to what has been found in other teleosts [10-11[18][19][26],26], Elovl4b proteins in both species were able to elongate 24:6n-3 up to 32:6n-3, a VLC-PUFA found in retinal phosphatidylcholine in fish [27-28][27][28]. Thus, this specific activity of Elovl4b proteins, along with the above mentioned presence of 32:6n-3 in fish retina, is coherent with the tissue expression results obtained for both species, in which Elovl4b transcripts were mostly found in the eye suggesting that, similarly to what has been described in other teleosts like Thunnus. thynnus [26], D. rerio [9], Acanthopagrus schlegelli [11][19], Oncorhynchus mykiss [23], S. salar [24] [24] or Epinephelus coioides [29], this is a major tissue for VLC-PUFA biosynthesis.
The quantitative expression results confirmed previous evidences of a differential elovl4a and elovl4b tissue-specific expression pattern [12[10][30],30], with elovl4a being mostly expressed in fish brain [9-11[9][18][19][23],23], and elovl4b in eye [9,11,23-24,26,29][9][19][23][24][26][29]. This results suggest a role of these enzymes in the local biosynthesis and incorporation of VLC-FA in fish neural tissues. This is in agreement with what is known in mammals [8], in which VLC-PUFA are key functional components, essential for the development and cell protection, of neural tissues such as those found in retina or brain [7,31-32][7][31][32]. Moreover, as previously described [12][10], retinogenesis in gilthead seabream and Senegalese sole larvae is clearly synchronized with an increase in expression of both elovl4 genes. Consequently, as described in mammals [33], alterations in VLC-PUFA biosynthesis could negatively impact visual acuity and disrupt brain functionality, jeopardizing the normal development of fish. This is particularly relevant in visual predators such as gilthead seabream and Senegalese sole, which previously showed a differential elovl4 expression in larvae and postlarvae according to the VLC-PUFA putative needs associated with each life-stage and LC-PUFA dietary availability [12,30][10][30]. Thus, the application of this knowledge is of special relevance during early larval development, particularly in species with high commercial interest for aquaculture production, as is the case of gilthead seabream and Senegalese sole, and should be kept in mind in feeding protocols during hatchery rearing.
References
- Monroig, Ó.; Tocher, D.R.; Castro, L.F.C. Polyunsaturated Fatty Acid Biosynthesis and Metabolism in Fish. In Polyunsaturated Fatty Acid Metabolism; Burdge, G.C., Ed.; Elsevier: Amsterdam, Netherlands, 2018; pp. 31-60.
- Castro, L.F.C.; Tocher, D.R.; Monroig, Ó. Long-chain polyunsaturated fatty acid biosynthesis in chordates: insights into the evolution of Fads and Elovl gene repertoire. Lipid Res. 2016, 62, 25-40.
- Guillou, H.; Zadravec, D.; Martin, P.G.; Jacobsson, A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: insights from transgenic mice. Lipid Res. 2010, 49, 186-199.
- Jakobsson, A.; Westerberg, R.; Jacobsson, A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Lipid Res. 2006, 45, 237-249.
- Li, Y.; Wen, Z.; You, C.; Xie, Z.; Tocher, D.R.; Zhang, Y.; Wang, S.; Li, Y. Genome wide identification and functional characterization of two LC-PUFA biosynthesis elongase (elovl8) genes in rabbitfish (Siganus canaliculatus). Aquaculture 2020, 735127.
- Oboh, A. Investigating the long-chain polyunsaturated fatty acid biosynthesis of the African catfish Clarias gariepinus (Burchell, 1822). PhD thesis. University of Stirling. Stirling. 2018.
- Agbaga, M.P.; Mandal, M.N.A.; Anderson, R.E. Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein. Lipid Res. 2010, 51, 1624-1642.
- Deák, F.; Anderson, R.E.; Fessler, J.L.; Sherry, D.M. Novel cellular functions of very long chain-fatty acids : Insight from ELOVL4 mutations. Cell. Neurosci. 2019, 13, 428.
- Monroig, Ó.; Rotllant, J.; Cerdá-Reverter, J.M.; Dick, J.R.; Figueras, A.; Tocher, D.R. Expression and role of Elovl4 elongases in biosynthesis of very long-chain fatty acids during zebrafish Danio rerio early embryonic development. Biophys. Acta-Mol. Cell Biol. Lipids 2010,1801, 1145-1154.
- Oboh, A.; Navarro, J.C.; Tocher, D.R.; Monroig, Ó. Elongation of very long-chain (>C24) fatty acids in Clarias gariepinus: cloning, functional characterization and tissue expression of elovl4 Lipids 2017, 52, 837-848.
- Jin, M.; Monroig, Ó.; Navarro, J.C.; Tocher, D.R.; Zhou, Q.C. Molecular and functional characterisation of two elovl4 elongases involved in the biosynthesis of very long-chain (>C24) polyunsaturated fatty acids in black seabream Acanthopagrus schlegelii. Biochem. Physiol. Part B Biochem. Mol. Biol. 2017, 212, 41-50.
- Torres, M.; Navarro, J.C.; Varó, I.; Agulleiro, M.J.; Morais, S.; Monroig, Ó.; Hontoria, F. Expression of genes related to long-chain (C18-22) and very long-chain (> C24) fatty acid biosynthesis in gilthead seabream (Sparus aurata) and Senegalese sole (Solea senegalensis) larvae : investigating early ontogeny and nutritional regulation. Aquaculture 2020, 520, 734949.
- Seiliez, I.; Panserat, S.; Corraze, G.; Kaushik, S.; Bergot, P. Cloning and nutritional regulation of a Δ6-desaturase-like enzyme in the marine teleost gilthead seabream (Sparus aurata). Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 135, 449-460.
- Zheng, X.; Seiliez, I.; Hastings, N.; Tocher, D.R.; Panserat, S.; Dickson, C.A.; Bergot, P.; Teale, A.J. Characterization and comparison of fatty acyl Δ6 desaturase cDNAs from freshwater and marine teleost fish species. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 139, 269-279.
- Agaba, M.K.; Tocher, D.R.; Zheng,X.; Dickson, C.A.; Dick, J.R.; Teale, A.J. Cloning and functional characterisation of polyunsaturated fatty acid elongases of marine and freshwater teleost fish. Biochem. Physiol. Part B Biochem. Mol. Biol. 2005, 142, 342-352.
- Morais, S.; Castanheira, F.; Martinez-Rubio, L.; Conceição, L.E.C.; Tocher, D.R. Long-chain polyunsaturated fatty acid synthesis in a marine vertebrate: Ontogenetic and nutritional regulation of a fatty acyl desaturase with Δ4 activity. Biophys. Acta - Mol. Cell Biol. Lipids 2012, 1821, 660-671.
- Sprecher, Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 2000, 1486, 219-231.
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I; Lu, F.; Marchler, G.H.; Song, J.S.; Thanki, N.; Wang, Z.; Yamashita, R.A.; Zhang, D.; Zheng, C.; Geer, L.Y.; Gwadz, M. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017. 4, D200-D203.
- Cook, H.W.; Mcmaster, C.R. Fatty acid desaturation and chain elongation in eukaryotes. In Biochemistry of Lipids, lipoproteins and membranes, 4th; Vance, D.E.; Vance, J.E., Eds.; Elsevier: Amsterdam, Netherlands, 2002, 36, 181-204.
- Yan, J.; Liang, X.; Cui, Y.; Cao, X.; Gao, J. Elovl4 can effectively elongate C18 polyunsaturated fatty acids in loach Misgurnus anguillicaudatus. Biophys. Res. Commun. 2018, 495, 2637-2642.
- Kabeya, N.; Yamamoto, Y.; Cummins, S.F.; Elizur, A.; Yazawa, R.; Takeuchi, Y.; Haga, Y.; Satoh, S.; Yoshizaki, G. Polyunsaturated fatty acid metabolism in a marine teleost, Nibe croaker Nibea mitsukurii: Functional characterization of Fads2 desaturase and Elovl5 and Elovl4 elongases. Biochem. Physiol. Part B Biochem. Mol. Biol. 2015, 188, 37-45.
- Monroig, Ó.; Webb, K.; Ibarra-Castro, L.; Holt, G.J.; Tocher, D.R. Biosynthesis of long-chain polyunsaturated fatty acids in marine fish: Characterization of an Elovl4-like elongase from cobia Rachycentron canadum and activation of the pathway during early life stages. Aquaculture 2011,312, 145-153.
- Zhao, N.; Monroig, Ó.; Navarro, J.C.; Xiang, X.; Li, Y.; Du, J.; Li, J.; Xu, W.; Mai, K.; Ai, Q. Molecular cloning, functional characterization and nutritional regulation of two elovl4b elongases from rainbow trout (Oncorhynchus mykiss). Aquaculture 2019, 511, 734221.
- Carmona-Antoñanzas, G.; Monroig, Ó.; Dick, J.R.; Davie, A.; Tocher, D.R. Biosynthesis of very long-chain fatty acids (C>24) in Atlantic salmon: Cloning, functional characterisation, and tissue distribution of an Elovl4 elongase. Biochem. Physiol. Part B Biochem. Mol. Biol. 2011, 159, 122-129.
- Suh, M.; Clandinin, M.T. 20:5n-3 but not 22:6n-3 is a Preferred Substrate for Synthesis of n-3 Very-Long- Chain Fatty Acids (C24-C36) in Retina. Eye Res. 2005, 30, 959-968.
- Betancor, M.B.; Oboh, A.; Ortega, A.; Mourente, G.; Navarro, J.C.; de la Gandara, F.; Tocher D.R.; Monroig, Ó. Molecular and functional characterisation of a putative elovl4 gene and its expression in response to dietary fatty acid profile in Atlantic bluefin tuna (Thunnus thynnus). Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 240, 110372.
- Monroig, Ó.; Hontoria, F.; Varó, I.; Tocher, D.R.; Navarro, J.C. Biosynthesis of very long -chain (>24C) polyunsaturated fatty acids in juveniles of gilthead seabream (Sparus aurata). In the 17th International Symposium on Fish Nutrition and Feeding, Sun Valley, USA, 2016.
- Garlito, B.; Portolés, T.; Niessen, W.M.A.; Navarro, J.C.; Hontoria, F.; Monroig, Ó.; Varó, I.; Serrano, R. Identification of very long-chain (>C24) fatty acid methyl esters using gas chromatography coupled to quadrupole/time-of-flight mass spectrometry with atmospheric pressure chemical ionization source. Chim. Acta. 2019, 1051, 103-109.
- Li, S.; Monroig, Ó.; Navarro, J.C.; Yuan, Y.; Xu, W.; Mai, K.; Tocher, D.R.; Ai, Q. Molecular cloning and functional characterization of a putative Elovl4 gene and its expression in response to dietary fatty acid profiles in orange-spotted grouper Epinephelus coioides. Res. 2017, 48, 537-552.
- Torres, M.; Navarro, J.C.; Varó, I.; Monroig, Ó.; Hontoria, F. Nutritional regulation of genes responsible for long-chain (C20-24) and very long-chain (> C24) polyunsaturated fatty acid biosynthesis in post-larvae of gilthead seabream (Sparus aurata) and Senegalese sole (Solea senegalensis). Aquaculture 2020, 735314.
- Bazan, N.G. Docosanoids and elovanoids from omega-3 fatty acids are pro-homeostatic modulators of inflammatory responses, cell damage and neuroprotection. Aspects Med. 2018, 64, 18-33.
- Agbaga, M.P.; Brush, R.S.; Mandal, M.N.A.; Henry, K.; Elliott, M.H.; Anderson, R.E. Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids. Natl. Acad. Sci. 2008, 105, 12843-12848.
- Bennett, L.D.; Brush, R.S.; Chan, M.; Lydic, T.A.; Reese, K.; Reid, G. E.; Busik, J.V; Elliott, M.H; Anderson, R.E. Effect of reduced retinal VLC-PUFA on rod and cone photoreceptors. Invest. Ophthalmol. Vis. Sci. 2014, 55, 3150-3157.
References
- Monroig, Ó.; Tocher, D.R.; Castro, L.F.C. Polyunsaturated Fatty Acid Biosynthesis and Metabolism in Fish. In Polyunsaturated Fatty Acid Metabolism; Burdge, G.C., Ed.; Elsevier: Amsterdam, Netherlands, 2018; pp. 31-60.
- L Filipe C Castro; D.R. Tocher; Óscar Monroig; Long-chain polyunsaturated fatty acid biosynthesis in chordates: Insights into the evolution of Fads and Elovl gene repertoire. Progress in Lipid Research 2016, 62, 25-40, 10.1016/j.plipres.2016.01.001.
- Hervé Guillou; Damir Zadravec; Pascal G. P. Martin; Anders Jacobsson; The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Progress in Lipid Research 2010, 49, 186-199, 10.1016/j.plipres.2009.12.002.
- Andreas Jakobsson; Rolf Westerberg; Anders Jacobsson; Fatty acid elongases in mammals: Their regulation and roles in metabolism. Progress in Lipid Research 2006, 45, 237-249, 10.1016/j.plipres.2006.01.004.
- Li, Y.; Wen, Z.; You, C.; Xie, Z.; Tocher, D.R.; Zhang, Y.; Wang, S.; Li, Y. Genome wide identification and functional characterization of two LC-PUFA biosynthesis elongase (elovl8) genes in rabbitfish (Siganus canaliculatus). Aquaculture 2020, 735127.
- Oboh, A. Investigating the long-chain polyunsaturated fatty acid biosynthesis of the African catfish Clarias gariepinus (Burchell, 1822). PhD thesis. University of Stirling. Stirling. 2018.
- Martin-Paul Agbaga; Nawajes A. Mandal; Robert E. Anderson; Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein. Journal of Lipid Research 2010, 51, 1624-1642, 10.1194/jlr.R005025.
- Ferenc Deák; Robert E. Anderson; Jennifer L. Fessler; David M. Sherry; Novel Cellular Functions of Very Long Chain-Fatty Acids: Insight From ELOVL4 Mutations.. Frontiers in Cellular Neuroscience 2019, 13, 428, 10.3389/fncel.2019.00428.
- Óscar Monroig; J. Rotllant; José Miguel Cerdá-Reverter; James R. Dick; Antonio Figueras; D.R. Tocher; Expression and role of Elovl4 elongases in biosynthesis of very long-chain fatty acids during zebrafish Danio rerio early embryonic development. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2010, 1801, 1145-1154, 10.1016/j.bbalip.2010.06.005.
- Torres, M.; Navarro, J.C.; Varó, I.; Agulleiro, M.J.; Morais, S.; Monroig, Ó.; Hontoria, F. Expression of genes related to long-chain (C18-22) and very long-chain (> C24) fatty acid biosynthesis in gilthead seabream (Sparus aurata) and Senegalese sole (Solea senegalensis) larvae : investigating early ontogeny and nutritional regulation. Aquaculture 2020, 520, 734949.
- Seiliez, I.; Panserat, S.; Corraze, G.; Kaushik, S.; Bergot, P. Cloning and nutritional regulation of a Δ6-desaturase-like enzyme in the marine teleost gilthead seabream (Sparus aurata). Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 135, 449-460.
- X. Zheng; I. Seiliez; N. Hastings; D.R. Tocher; Stéphane Panserat; C.A. Dickson; P. Bergot; A.J. Teale; Characterization and comparison of fatty acyl Δ6 desaturase cDNAs from freshwater and marine teleost fish species. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 2004, 139, 269-279, 10.1016/j.cbpc.2004.08.003.
- Agaba, M.K.; Tocher, D.R.; Zheng,X.; Dickson, C.A.; Dick, J.R.; Teale, A.J. Cloning and functional characterisation of polyunsaturated fatty acid elongases of marine and freshwater teleost fish. Biochem. Physiol. Part B Biochem. Mol. Biol. 2005, 142, 342-352.
- Sofia Morais; Filipa Castanheira; Laura Martinez-Rubio; Luis Conceicao; D.R. Tocher; Long chain polyunsaturated fatty acid synthesis in a marine vertebrate: Ontogenetic and nutritional regulation of a fatty acyl desaturase with Δ4 activity. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2012, 1821, 660-671, 10.1016/j.bbalip.2011.12.011.
- Sprecher, Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 2000, 1486, 219-231.
- Aron Marchler-Bauer; Yu Bo; Lianyi Han; Jane He; Christopher J. Lanczycki; Shennan Lu; Farideh Chitsaz; Myra K. Derbyshire; Renata C. Geer; Noreen R. Gonzales; Marc Gwadz; David I. Hurwitz; Fu Lu; Gabriele H. Marchler; James S. Song; Narmada Thanki; Zhouxi Wang; Roxanne A. Yamashita; Dachuan Zhang; Chanjuan Zheng; Lewis Y. Geer; Stephen H. Bryant; CDD/SPARCLE: functional classification of proteins via subfamily domain architectures.. Nucleic Acids Research 2016, 45, D200-D203, 10.1093/nar/gkw1129.
- Harold W. Cook; Fatty acid desaturation and chain elongation in eukaryotes. Structural and Evolutionary Genomics: Natural Selection in Genome Evolution 1996, 31, 129-152, 10.1016/s0167-7306(08)60512-8.
- Oboh, A.; Navarro, J.C.; Tocher, D.R.; Monroig, Ó. Elongation of very long-chain (>C24) fatty acids in Clarias gariepinus: cloning, functional characterization and tissue expression of elovl4 Lipids 2017, 52, 837-848.
- Min Jin; Óscar Monroig; Juan C. Navarro; D.R. Tocher; Qicun Zhou; Molecular and functional characterisation of two elovl4 elongases involved in the biosynthesis of very long-chain (> C 24 ) polyunsaturated fatty acids in black seabream Acanthopagrus schlegelii. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 2017, 212, 41-50, 10.1016/j.cbpb.2017.06.008.
- Jie Yan; Xiao Liang; Yun Cui; Xiaojuan Cao; Jian Gao; Elovl4 can effectively elongate C18 polyunsaturated fatty acids in loach Misgurnus anguillicaudatus. Biochemical and Biophysical Research Communications 2018, 495, 2637-2642, 10.1016/j.bbrc.2017.12.123.
- Naoki Kabeya; Yoji Yamamoto; Scott F. Cummins; Abigail Elizur; Ryosuke Yazawa; Yutaka Takeuchi; Yutaka Haga; Shuichi Satoh; Goro Yoshizaki; Polyunsaturated fatty acid metabolism in a marine teleost, Nibe croaker Nibea mitsukurii: Functional characterization of Fads2 desaturase and Elovl5 and Elovl4 elongases. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 2015, 188, 37-45, 10.1016/j.cbpb.2015.06.005.
- Óscar Monroig; Ken Webb; Leonardo Ibarra-Castro; G. Joan Holt; D.R. Tocher; Kenneth Webb; Biosynthesis of long-chain polyunsaturated fatty acids in marine fish: Characterization of an Elovl4-like elongase from cobia Rachycentron canadum and activation of the pathway during early life stages. Aquaculture 2011, 312, 145-153, 10.1016/j.aquaculture.2010.12.024.
- Zhao, N.; Monroig, Ó.; Navarro, J.C.; Xiang, X.; Li, Y.; Du, J.; Li, J.; Xu, W.; Mai, K.; Ai, Q. Molecular cloning, functional characterization and nutritional regulation of two elovl4b elongases from rainbow trout (Oncorhynchus mykiss). Aquaculture 2019, 511, 734221.
- Greta Carmona-Antoñanzas; Óscar Monroig; James R. Dick; Andrew Davie; D.R. Tocher; Biosynthesis of very long-chain fatty acids (C>24) in Atlantic salmon: Cloning, functional characterisation, and tissue distribution of an Elovl4 elongase. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 2011, 159, 122-129, 10.1016/j.cbpb.2011.02.007.
- Miyoung Suh; M. Thomas Clandinin; 20:5n-3 but not 22:6n-3 is a Preferred Substrate for Synthesis of n-3 Very-Long- Chain Fatty Acids (C24–C36) in Retina. Current Eye Research 2005, 30, 959-968, 10.1080/02713680500246957.
- Betancor, M.B.; Oboh, A.; Ortega, A.; Mourente, G.; Navarro, J.C.; de la Gandara, F.; Tocher D.R.; Monroig, Ó. Molecular and functional characterisation of a putative elovl4 gene and its expression in response to dietary fatty acid profile in Atlantic bluefin tuna (Thunnus thynnus). Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 240, 110372.
- Monroig, Ó.; Hontoria, F.; Varó, I.; Tocher, D.R.; Navarro, J.C. Biosynthesis of very long -chain (>24C) polyunsaturated fatty acids in juveniles of gilthead seabream (Sparus aurata). In the 17th International Symposium on Fish Nutrition and Feeding, Sun Valley, USA, 2016.
- B. Garlito; T. Portolés; Wilfried Niessen; Juan C. Navarro; F. Hontoria; Ó. Monroig; I. Varó; R. Serrano; Identification of very long-chain (>C24) fatty acid methyl esters using gas chromatography coupled to quadrupole/time-of-flight mass spectrometry with atmospheric pressure chemical ionization source. Analytica Chimica Acta 2019, 1051, 103-109, 10.1016/j.aca.2018.11.001.
- Songlin Li; Óscar Monroig; Juan C. Navarro; Yuhui Yuan; Wei Xu; Kangsen Mai; D.R. Tocher; Qinghui Ai; Molecular cloning and functional characterization of a putativeElovl4gene and its expression in response to dietary fatty acid profiles in orange-spotted grouperEpinephelus coioides. Aquaculture Research 2015, 48, 537-552, 10.1111/are.12902.
- Torres, M.; Navarro, J.C.; Varó, I.; Monroig, Ó.; Hontoria, F. Nutritional regulation of genes responsible for long-chain (C20-24) and very long-chain (> C24) polyunsaturated fatty acid biosynthesis in post-larvae of gilthead seabream (Sparus aurata) and Senegalese sole (Solea senegalensis). Aquaculture 2020, 735314.
- Nicolas G. Bazan; Docosanoids and elovanoids from omega-3 fatty acids are pro-homeostatic modulators of inflammatory responses, cell damage and neuroprotection. Molecular Aspects of Medicine 2018, 64, 18-33, 10.1016/j.mam.2018.09.003.
- Martin-Paul Agbaga; Richard S. Brush; Nawajes A. Mandal; Kimberly Henry; Michael H. Elliott; Robert E. Anderson; Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids.. Proceedings of the National Academy of Sciences 2008, 105, 12843-8, 10.1073/pnas.0802607105.
- Lea D. Bennett; Richard S. Brush; Michael Chan; Todd A. Lydic; Kristen Reese; Gavin Reid; Julia V. Busik; Michael H. Elliott; Robert E. Anderson; Effect of Reduced Retinal VLC-PUFA on Rod and Cone Photoreceptors. Investigative Opthalmology & Visual Science 2014, 55, 3150-3157, 10.1167/iovs.14-13995.