The synthesis of fluorine-containing small molecules has had numerous benefits of improving the quality and efficiency of many applications of these compounds. Compounds such as derivatives of boron-dipyrromethene (BODIPY) typically demonstrate signal wavelengths around 500 nm and 600 nm; however, it is more favorable to see these signals closer to 700 nm to improve fluorophore applications. For this reason, recent studies described further in the text demonstrate the applications of adding fluorine-based substituents and phenyl rings to the chemical designs, thus redshifting absorption and fluorescence wavelengths.
- fluorine chemistry
- optical fluorophores
- PET imaging
- PDT
- contrast agents
1. Fluorinated BODIPY Dyes
Martinez Espinoza et al. studied the synthesis and effects of branched fluorinated chains off a BODIPY scaffold [1]. The synthetic design focused on the fluorinated chain placement in two different positions. One position was associated with an alkoxy group of the meso position of the dye, as seen in compound 3 (Scheme 1). The scheme begins with a substitution reaction between 4-hydroxybenzaldehyde reacting with fluorinated mesylate (Rf-OMs) under basic conditions to generate aldehyde 1. Using this intermediate, a fluorinated BODIPY 2, is formed in three steps typical of BODIPY scaffold formation. The following step is iodination to add iodine atoms to the BODIPY core; this modification is described to promote singlet oxygen generation in the final compound. The step after iodination adds two equivalents of p-tolualdehyde to the scaffold through Knoevenagel condensation to extend the conjugation of the final compound 3 with a yield of 49%.
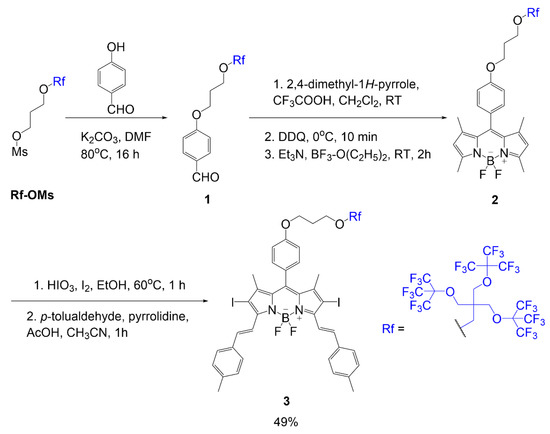
Scheme 1. BODIPY with multi-branched fluorinated chains on meso phenyl [1].
The other placement was a substitution of fluorine atoms at the boron center with alkoxy groups containing the fluorinated chain as seen in compounds 4 and 5 (Figure 31) [1]. In these dyes, an iodination step occurs to add iodine atoms to the BODIPY core to promote singlet oxygen generation. Both compounds contain a substitution to gain the fluorinated branches; however, compound 5 undergoes Knoevenagel condensation to extend conjugation before substituting the fluorine atoms. The structural differences in compounds 4 and 5 compared to compounds 2 and 3 are important to consider when determining the bathochromic shift caused by the substitution of the fluorinated chains because it is important to understand the impact of the fluorinated chains with and without the extended conjugation.
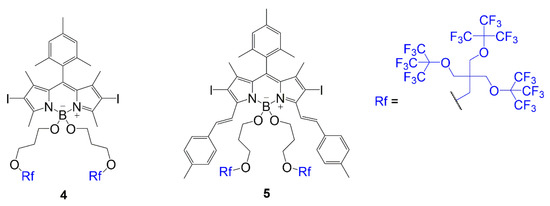
Figure 31. BODIPY with multi-branched fluorinated chains attached to boron center [1].
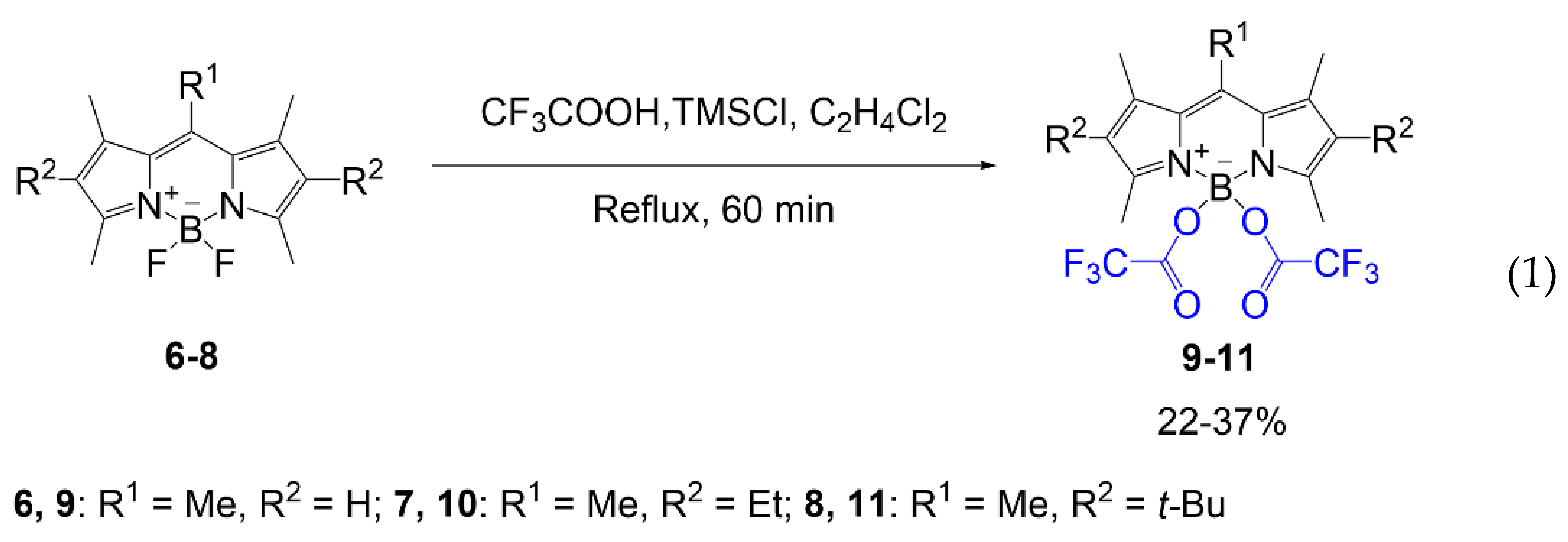
Another example of fluorine substitution on the boron center is viewed in the synthesis conducted by Duran-Sampedro et al. (Equation (1)). Trifluoroacetoxy groups replace both fluorine atoms [2]. The synthesis was accomplished to demonstrate the enhanced fluorescent effect of trifluoroacetoxy compared to a BODIPY with a standard core. This substitution method requires reagent TMSOCOCF3, which is synthesized using trifluoroacetic acid and TMSCl in 1,2-dichloroethane, to react with individual compounds 6–8. The reaction affords solid compounds 9–11 (yields 22–37%) with fluorine demonstrating halogenic effects in the boron center of the BODIPY.
In the next example, the aza-BODIPY 16 was synthesized as a trimodal contrast agent designed for fluorescence imaging, photoacoustic imaging, and 19F MRI [3]. Of the listed modalities, the BODIPY core demonstrates extended conjugation for enhancing fluorescence and photoacoustic imaging, and the trifluoromethyl groups on the aromatic ring are designed for 19F MRI functionality. In Scheme 2, the synthesis of contrast agent 16 begins with the ring closure of the BODIPY using compound 12 with ammonium acetate in ethanol under microwave conditions; the second step completes the closure using a boron-source to yield BODIPY 13. A nucleophilic substitution reaction occurs between compound 13 and 3-bromo-propyne to form compound 14. Through click chemistry, alkyne 14 forms a five-membered triazole ring with azide 15 to yield final compound 16 in a good yield of 41%.
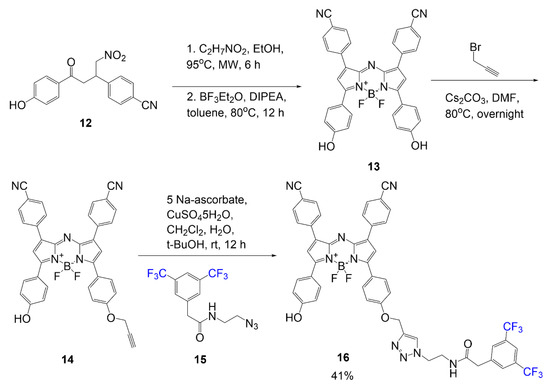
Scheme 2. Synthesis of fluorinated trimodal BODIPY 16 [3].
Scheme 3 illustrates the synthesis of fluorinated BODIPY synthesized using a gold catalyst for the cycloisomerization of the fluorine-containing pyrrole precursor [4]. In previous literature, research groups have been studying the use of aromatic groups to extend conjugation of BODIPY dyes [5]. In this study, they synthesize pyrrole rings with aromatic rings containing trifluoromethyl group(s). The first reaction shown in Scheme 3 is the two steps done on compound 17 or 18 to form the pyrrole 19 or 20. The first step demonstrates the use of the gold catalyst in ionic liquid, and the second step shows the basic conditions to finalize the ring closure. After generating the relevant pyrrole, the BODIPY scaffold is synthesized using the pentafluorobenzaldehyde and pyrrole 19 or 20 with BODIPY reagents: chloroanile, BF3·Et2O, and DIPEA; this reaction generates product 21 or 22 in good yield 68–70%.
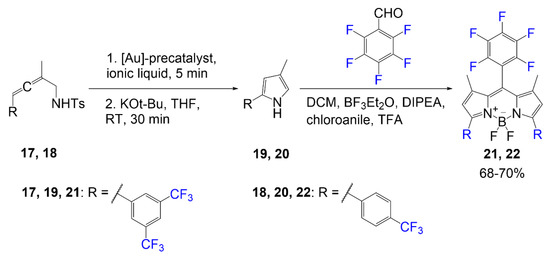
Scheme 3. Fluorinated BODIPY synthesized via gold catalyst [4].
In an effort to design a BODIPY dye with higher photostability, Hecht et al. synthesized a series of fluorinated BODIPY dyes and fluorinated BODIPY dyes with extended conjugation of the alpha-3 and alpha-5 positions [6]. As shown in Scheme 4, compound 25 was synthesized using the general BODIPY procedure to obtain compound 24, followed by a Knoevenagel condensation using a fluorine-containing benzaldehyde. The Knoevenagel condensation is achieved utilizing a benzaldehyde derivative and a piperidinium acetate catalyst. This conjugation extension is used to shift BODIPY absorption values to higher wavelengths. The yield reported for the synthesis of dye 25 is 21%.
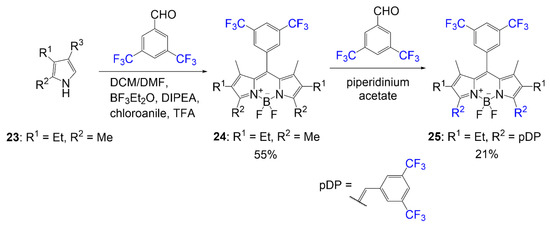
Scheme 4. Synthesis of BODIPY containing fluorinated aryl groups [6].
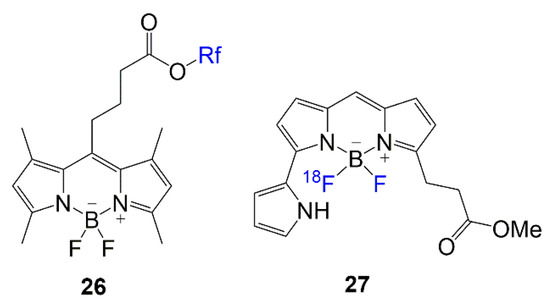
Lastly, in Figure 12, the chemical structures of BODIPY compounds 26 and 27 are displayed. These compounds are relevant in recent literature for their biological applications. The design for dye 26 is similar to the rationale of compounds 2–5, with multi-branched fluorinated chains being the focus of the molecule [7]. The synthesis of dye 27 mostly addresses the fluorine exchange to generate the 18F-labeled BODIPY to introduce the PET modality to the scaffold for brain imaging [8].
2. Optical Properties of Fluorinated BODIPY
BODIPY compounds typically demonstrate absorption spectra with a peak around 510 nm, typically more intense and relevant as a visible/NIR contrast agent. Newer dyes in literature have aryl groups in different positions of the BODIPY dye to afford shifts in wavelength that will improve in vivo applications. The most common is having a phenyl group at the meso position. Studies were conducted to view the effect fluorine atoms have on BODIPY when fluorine is observed in the meso phenyl at different placements [9]. These studies have indicated increasing absorbance values associated with increasing fluorine atoms in the molecule. They have also noted shifts in absorbance wavelength due to the position of the fluorine atoms in proximity to the BODIPY core. The absorption shift and increasing quantum yields are typically observed in compounds with halogens; however, the halogen’s position will affect the degree to which the increase is experienced. Table 1 indicates the optical properties of BODIPY fluorophores shown in Scheme 1, Scheme 2, Scheme 3 and Scheme 4, and Equation (1) with fluorine-containing functional groups/atoms at differing positions.
Table 1. Optical properties of selected fluorine containing BODIPY dyes.
| Dye | λAbs (nm) | λEm (nm) | ε (M−1 cm−1) | Stokes Shift (nm) | ΦF | Solvent | Applications |
|---|---|---|---|---|---|---|---|
| 2 | 531 | 550 | 103,000 | 21 | 0.05 | THF | 19F MRI |
| 3 | 645 | 657 | 99,000 | 12 | 0.04 | THF | 19F MRI |
| 4 | 531 | 551 | 138,000 | 20 | 0.04 | THF | 19F MRI |
| 5 | 640 | 653 | 104,000 | 13 | 0.27 | THF | 19F MRI |
| 9 | 502 | 509 | 102,000 | 7 | 0.95 | Cyclohexane | Optical ** |
| 10 | 525 | 541 | 78,000 | 16 | 0.94 | Cyclohexane | Optical ** |
| 11 | 532 | 571 | 54,000 | 39 | 0.76 | Cyclohexane | Optical ** |
| 16 | 735 | 780 | 59,815 | 45 | 0.42 | DMSO | 19F MRI/NIR/PA |
| 21 | 556 | 587 | 46,600 | 31 | 0.91 | Acetonitrile | Optical ** |
| 22 | 550 | 587 | 58,100 | 37 | 0.94 | Acetonitrile | Optical ** |
| 24 | 528 | 541 | 56,500 | 13 | 0.72 | Acetonitrile | NIR * |
| 25 | 637 | 650 | 70,500 | 13 | 0.80 | Acetonitrile | NIR * |
| 26 | 501 | 513 | --- | 12 | --- | DCM | 19F MRI |
| 27 | 580 | 590 | --- | 10 | --- | MeOH | PET |
* NIR used for fluorescence imaging compounds; ** Optical used for compounds used for fluorescence studies in visible range.
Substitutions of fluorine on the boron of the BODIPY for trifluoroacetoxy causes a shift in peak absorbance wavelength [22]. Compared to the compounds created from the substitution of fluorine for acetoxy groups, compounds 9–11 demonstrate slightly blueshifted absorbance values, 502–532 nm; however, it demonstrates redshifted optical values compared to precursors 6–8 detailed in the literature. Quantum yield of compounds 9–11 does increase from the presence of fluorine in the trifluoroacetoxy groups compared to both the commercially available BODIPY 6–8 and the nonfluorinated versions of these fluorophores.
In the example of the trimodal BODIPY, compound 16 was synthesized [3]. As expected, the addition of the component containing the trifluoromethyl groups did not afford a bathochromic shift as seen in other compounds; however, this was not expected due to the proximity of the fluorine to the BODIPY core. A significant increase in molar absorptivity, 59,815 M−1 cm−1, and quantum yield 42% were notably observed, like other fluorine-containing compounds.
Compounds 21 and 22 are similar in structure to compound 25. They contain a fluorinated phenyl group in the meso position, either pentafluorophenyl or trifluoromethyl groups on phenyl. When comparing compounds 21 and 22 to compound 25, the fluorinated phenyl affords a 10–16 nm shift. Most of the newer compounds in literature utilize a combination of increasing the conjugation of the BODIPY and increasing the number of fluorine atoms in the molecule to increase molecule absorbance around 30–200 nm. This shift in the wavelength offers the potential to push the synthesis of BODIPY compounds into the NIR optical window to improve the properties of these molecules applied as contrast agents without making them too large or complex.
3. Applications of Fluorinated BODIPY
Literature explores the lipophilic effect that is caused by fluorine atom in BODIPY dyes [9]. The fluorinated version of the BODIPY compounds is comparable to the version of the structure with hydrogen in the place of fluorine due to the compound’s size while offering electron-withdrawing halogenic effects to small molecules. This difference allows research to be done to compare hydrogen and fluorine atoms to explore the impact of fluorine atoms on BODIPY based on the quantity and position of atoms. Some of the most important studies to occur are the observations of fluorine substitutions on the center boron and observing fluorine effects on aromatic carbons.
Many well-known BODIPY dyes have been utilized for medical imaging. BODIPY dyes can be used as fluorescent switches and sensitizers for metal/pH sensing and being designed for specific cell or biomolecule targeting [10]. Although BODIPY compounds are typically hydrophobic compounds, which is a disadvantage for them to be utilized for biological applications, the addition and placement of the fluorine atoms can help overcome hydrophobicity [5]. In the example of Figure 23, dye 16 is used as a trimodal contrast agent because, in acidic conditions, the agent will exhibit fluorescent properties [3]. Fluorescence imaging alongside photoacoustic imaging (PAI) and 19FMRI allow for the activity of the compounds to be enhanced for tumor imaging and analysis. The fluorescence imaging shows the dye localizing to the tumor, while 19FMRI and PAI imaging show sharp signals for the tumor cells.
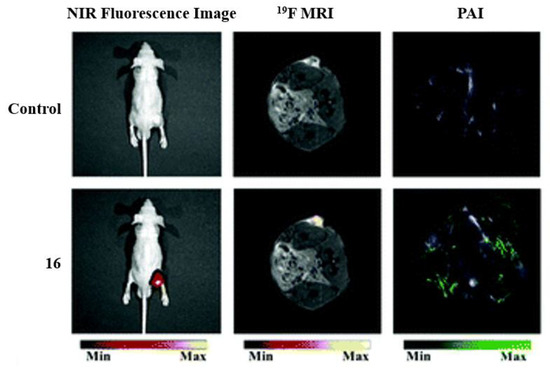
Figure 23. Applications of fluorinated trimodal compound 16, for in vivo imaging. Imaging of an A549 tumor in the mouse model. Before injection (control, top) and after injection (bottom) demonstrating the three imaging modalities: NIR fluorescence (left), 19FMRI (middle), and photoacoustic imaging (PAI) (right). Figure used with permission from [3]. Copyright 2019 Royal Society of Chemistry.
The introduction of fluorine atoms in these molecules shows improvements in the specificity of signal in certain forms of imaging. Figure 34 shows imaging comparing 19F signal to 1H signal in a mouse model injected with 100 mM of compound 26, and the intensity of the fluorine signal in one location is appreciable [7]. The signal shows relatively high intensity for the fluorophore pictured (left); this compound has a similar structure to BODIPY compounds 2–5 featuring the multi-branch fluorination (Scheme 1). This observation is important to consider since the 19F signal is very bright and localized to one area of the abdominal cavity, making it worth further investigating multi-branched fluorinated chains on BODIPYs.
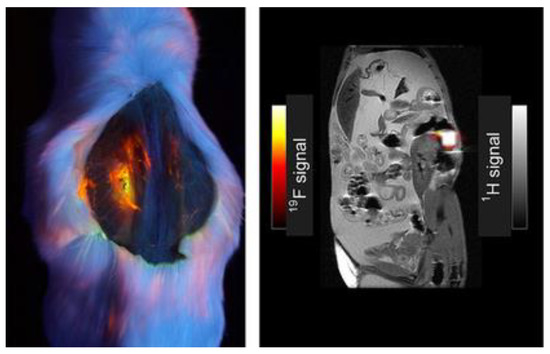
Figure 34. Dual fluorescence and 19F MRI, dye 26 (100 µL of 100 mM in DCM). Image under black-light excited at 365 nm of mouse post-mortem (left); grayscale image (right) demonstrating overlay of 1H and 19FMRI. Figure used with permission from [7]. Copyright 2016 John Wiley & Sons.
One of the most interesting uses of these fluorine-containing BODIPYs is its applications in Positron Emission Tomography (PET) imaging [8][11]. Figure 45 demonstrates imaging after injection of compound 27 into a mouse model. It is observed that strong signals are observed in the brain upon 2 min of injection and diminishes in the images after 30 min. Although this is a short amount of time for a dye, this is an impressive achievement as many groups are pursuing brain-targeting probes. Although other compounds possess the potential for this type of application, these BODIPYs with the active fluorine substituted onto the boron have shown some of the most reports for PET imaging data in the literature, making fluorine-exchange on the boron well-known in BODIPY synthesis [8]. This functionality continues to be a growing interest in BODIPY research with continued improvements in the solubility of these compounds and the penetration of the blood–brain barrier.
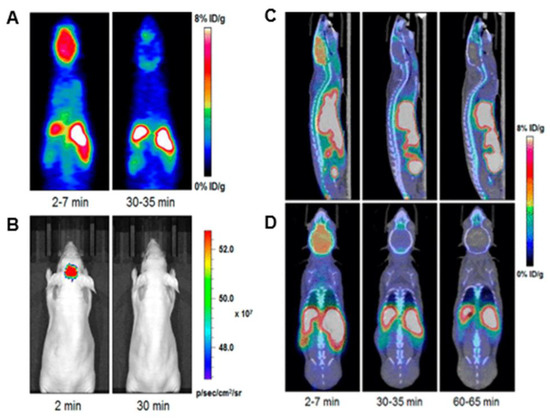
Figure 45. Radioactive fluorine exchange in BODIPY used for PET, compound 27. (A) PET imaging and (B) optical imaging was done at two intervals: 2 min and 30 min (0.1 GBq/µmol). (C,D) indicate MicroPET/CT images at the same intervals with an additional image at 60 min; (C) sagittal view and (D) coronal view (0.8–1 GBq/μmol). Figure used with permission from [8]. Copyright 2019 American Chemical Society.
Some literature recognizes the potential for fluorinated BODIPY compounds to be used in cell imaging due to their lipophilic character having the potential for cell membrane staining [4]. The literature addresses the tremendous potential fluorine atoms cause for fluorescence imaging through improvements in molar absorptivity, increased fluorescence efficiency, and increased lasing efficiency [2]. However, most of the literature emphasizes the improvements in optical properties above all else on the effects fluorine has on BODIPY.
References
- Martinez-Espinoza, M.I.; Sori, L.; Pizzi, A.; Terraneo, G.; Moggio, I.; Arias, E.; Pozzi, G.; Orlandi, S.; Dichiarante, V.; Metrangolo, P.; et al. BODIPY dyes bearing multibranched fluorinated chains: Synthesis, structural, and spectroscopic studies. Chem. A Eur. J. 2019, 25, 9078–9087.
- Durán-Sampedro, G.; Agarrabeitia, A.R.; Cerdán, L.; Pérez-Ojeda, M.E.; Costela, A.; García-Moreno, I.; Esnal, I.; Bañuelos, J.; Arbeloa, I.L.; Ortiz, M.J. Carboxylates versus Fluorines: Boosting the emission properties of commercial BODIPYs in liquid and solid media. Adv. Funct. Mater. 2013, 23, 4195–4205.
- Liu, L.; Yuan, Y.; Yang, Y.; McMahon, M.T.; Chen, S.; Zhou, X. A fluorinated aza-BODIPY derivative for NIR fluorescence/PA/19F MR tri-modality in vivo imaging. Chem. Commun. 2019, 55, 5851–5854.
- Lempke, L.; Fischer, T.; Bell, J.; Kraus, W.; Rurack, K.; Krause, N. Gold-catalyzed allene cycloisomerization for pyrrole synthesis: Towards highly fluorinated BODIPY dyes. Org. Biomol. Chem. 2015, 13, 3787–3791.
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932.
- Hecht, M.; Fischer, T.; Dietrich, P.; Kraus, W.; Descalzo, A.B.; Unger, W.E.S.; Rurack, K. Fluorinated boron-dipyrromethene (BODIPY) dyes: Bright and versatile probes for surface analysis. ChemistryOpen 2013, 2, 25–38.
- Huynh, A.M.; Müller, A.; Kessler, S.M.; Henrikus, S.; Hoffmann, C.; Kiemer, A.K.; Bücker, A.; Jung, G. Small BODIPY probes for combined dual 19F MRI and fluorescence imaging. ChemMedChem 2016, 11, 1568–1575.
- Kim, H.; Kim, K.; Son, S.-H.; Choi, J.Y.; Lee, K.-H.; Kim, B.-T.; Byun, Y.; Choe, Y.S. 18F-Labeled BODIPY dye: A potential prosthetic group for brain hybrid PET/optical imaging agents. ACS Chem. Neurosci. 2019, 10, 1445–1451.
- Alamiry, M.A.H.; Benniston, A.C.; Hagon, J.; Winstanley, T.P.L.; Lemmetyinen, H.; Tkachenko, N.V. The fluorine effect: Photophysical properties of borondipyrromethene (BODIPY) dyes appended at the meso position with fluorinated aryl groups. RSC Adv. 2012, 2, 4944–4950.
- Ziarani, G.M.; Moradi, R.; Lashgari, N.; Kruger, H.G. Chapter 5-BODIPY dyes. In Metal-Free Synthetic Organic Dyes; Ziarani, G.M., Moradi, R., Lashgari, N., Kruger, H.G., Eds.; Elsevier: Amsterdam, NL, USA, 2018; pp. 95–107.
- Chansaenpak, K.; Wang, H.; Wang, M.; Giglio, B.; Ma, X.; Yuan, H.; Hu, S.; Wu, Z.; Li, Z. Synthesis and evaluation of [18F]-ammonium BODIPY dyes as potential positron emission tomography agents for myocardial perfusion imaging. Chem. A Eur. J. 2016, 22, 12122–12129.