Starch is the second most abundantly available natural polymer in the world, after cellulose. If we add its biodegradability and non-toxicity to the natural environment, it becomes a raw material very attractive for the food and non-food industries. However, in the latter case, mainly due to the high hydrophilicity of starch, it is necessary to carry out many more or less complex operations and processes. One of the fastest growing industries in the last decade is the processing of biodegradable materials for packaging purposes. This is mainly due to awareness of producers and consumers about the dangers of unlimited production and the use of non-degradable petroleum polymers.
- starch
- modification
- packaging
- non-food applications
- biodegradable polymers
1. Introduction
Humanity increasingly understands that the earth is not an unlimited source of raw materials and that the overexploitation of natural resources causes a lot of damage to the environment. Everyone can agree that the world economy should be increasingly based on renewable raw materials and energy sources. The growing demand for new organic materials and energy demand encourages the search for cheap, widely available biodegradable raw materials and environmentally friendly methods of their processing [1]. It seems more and more certain that the way of dealing with or eliminating the problems of raw materials is through the use of natural polymers based on polysaccharides. Although the vast majority of carbohydrate polymers are used for food purposes, it is predicted that these proportions will change significantly in the coming decades in favor of the synthesis of new functional non-food materials.
The reduction of fossil-fuel resources and environmental problems have contributed to the development of compounds based on natural polymers that can replace traditional petrochemical polymers. Cheap, non-toxic, and biodegradable materials are sought [2]. One of them is starch, the main energy storage in plants and the second-most abundant renewable polysaccharide in the world after cellulose. Starch has long been popular as a raw material for the production of biodegradable packaging. However, the main disadvantage of starch as a potential packaging material is its strong hydrophilicity, high sensitivity to external factors, mainly moisture, brittleness, and very poor miscibility with hydrophobic synthetic polymers. A huge opportunity was seen in the thermoplastic starches (TPS), i.e., in amorphous and homogeneous forms, obtained under the influence of thermomechanical energy during extrusion [3]. This method of plasticizing starch, however, did not solve the problem of brittleness, sensitivity to external factors and poor mechanical properties compared to other polymeric materials. The situation tried to improve with the formation of blends (mixtures) of thermoplastic starch with synthetic, usually aliphatic polymers, for example, polylactide (PLA) or poly-ε-caprolactone (PCL). Still, there was a compatibility problem. The addition of external plasticizers or fillers only slightly increased the processing and usability of starch films [4]. Therefore, to expand the application potential, starch undergoes various types of modifications, from low-complex hydrothermal treatments (like the mentioned plasticization or extrusion) to multistage processes and reactions, using complex technologies [5][6]. Most of them were carried out for hydrophobization. Hydrophobic starch derivatives have usually been synthesized using classic organic/inorganic solvents such as dimethyl sulfoxide (DMSO), dimethylformamide (DMF),
-dimethylacetamide/lithium chloride (DMAc/LiCl), or pyridine. Mainly their flammability and high toxicity are the features that pose a serious threat during the washing and recovery process and thus can be a considerable and troublesome pollution for the natural environment [7]. For this reason, it has become important to search for a new type of media that is most compatible with the principles of the “green chemistry” as well as the principles of sustainable development. In the last decade, unconventional media (ionic liquids and supercritical CO
) and reaction techniques (microwave and ultrasound) have become increasingly popular [8]. Due to the mild reaction conditions and the fact that no harmful byproducts are generated, biocatalysts have also gained great importance in the modification of starch [9][10].
2. Structure and Properties of Starch
Chemically, starch is a homopolysaccharide. It is composed of one type of unit—α-
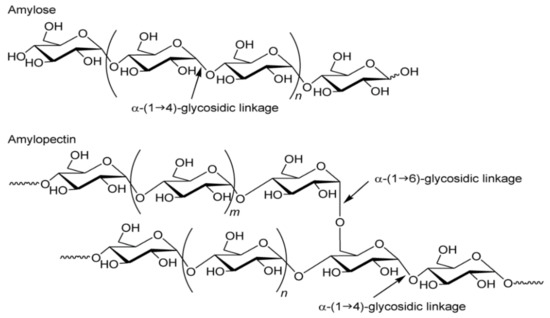
d-glucopyranose, occurring in the form of closed six-carbon rings, cocreating chain and branched forms of the polymer. The α-1,4 and α-1,6 glycosidic bonds dominate in the connections of the polymer glucose units [11]. Such bonds are formed as a result of the joining of neighboring α-D glucose molecules through the oxygen atom cocreating the hydroxyl group, with the release of the water molecule. Glucose polymerization in the starch molecule produces two polysaccharide fractions: linear amylose and branched amylopectin [12]. Both fractions consist of chains composed of α-
d-glucopyranose residues connected by α-1,4-glycosidic bonds, while the chains are connected with each other by α-1,6-glycosidic bonds, thus creating branches in polymers (
Figure 1). In principle, amylose is considered to be a typical long-chain linear fraction, but few branchings have been proven to occur in it. Both the branched and linear forms of amylose consist of long chains. The second fraction, amylopectin, is characterized by a high degree of branching (approx. 5% of the molecule) and relatively different chains, leading to a highly complex molecular structure. Amylopectin consists of long and short chains. Considering the branching points in addition to the length, we distinguish: the shortest A-chains, without branches, the B-chains that are branched by the A-chains or other B-chains, and the C-chains that contain the only reducing terminal residue and B-chains. The number of short chains as branches are larger than that of long one, but despite this, the size of amylopectin as an integrated structure of many chains is much larger than amylose [13,14].). In principle, amylose is considered to be a typical long-chain linear fraction, but few branchings have been proven to occur in it. Both the branched and linear forms of amylose consist of long chains. The second fraction, amylopectin, is characterized by a high degree of branching (approx. 5% of the molecule) and relatively different chains, leading to a highly complex molecular structure. Amylopectin consists of long and short chains. Considering the branching points in addition to the length, we distinguish: the shortest A-chains, without branches, the B-chains that are branched by the A-chains or other B-chains, and the C-chains that contain the only reducing terminal residue and B-chains. The number of short chains as branches are larger than that of long one, but despite this, the size of amylopectin as an integrated structure of many chains is much larger than amylose [13][14].
The occurrence of certain physicochemical features of starch depends, to a large extent, on the genetic conditions, species and varieties of plants from which it was isolated, as well as the conditions of cultivation of these plants. Furthermore, strong differences in physical and chemical properties are dictated by the differences in the content of non-polysaccharide components as well as the structure of starch grains [11].
Native starch is insoluble in cold water and most organic solvents, which is particularly associated with the presence of insoluble or sparingly soluble amylose fraction. As previously mentioned, when discussing non-polysaccharide starch ingredients, one of the above is water. Starchy grains can increase its amount through so-called reversible swelling, manifesting an increase in their volume of up to 30% [14]. Swelling is an exothermic process during which water molecules are absorbed into the amorphous zone, where they are bonded by hydrogen bonds with free hydroxyl groups of the glucose units in polymer chains. However, if the aqueous starch suspension is heated above a certain temperature, the starch granules swell spherically and become amorphous. The above thermal transition is referred to as starch gelatinization, the temperature of which is that of gelatinization [16]. It is a parameter with different values depending on the type of botanical starch. Moreover, using the term “gelatinization” temperature range is more appropriate because the gelatinization of grains depends on their size, from the largest to the smallest, thus gradually proceeding [17]. For example, for potato starch, this range is between 50–70 °C. Gelatinization is accompanied by other phenomena or processes such as loss of birefringence, increase in viscosity, and electrical conductivity. Over time, starch gruel may be subject to retrogradation. This is an irreversible process, manifesting itself in the formation of insoluble amylose aggregates precipitated in the form of dendrites. In other words, it is a type of secondary crystallization by restoring hydrogen bonds between hydroxyl groups accompanied by syneresis (dehydration: molecules get rid of water as a result of reducing and tightening intermolecular spaces).
Another important property of starch, in which it is hard to de facto explicitly include physical or chemical properties, is its ability to form inclusion complexes. The most famous of them is the amylose-iodine complex in the presence of iodide ions, where the internal space of the amylose helix is filled with iodine molecules. The resulting complex ion gives a characteristic dark-blue color, disappearing after the previous denaturation of the amylose chain, occurring, e.g., after heating or hydrolysis. Iodine complexation can be used as one of the faster methods for determining the degree of the depolymerisation of amylose (in shorter chains, the complexes turn red or purple). Amylose can also be complex with other compounds, e.g., organic, to form a characteristic crystallographic structure called “V”. The way of complexing depends on how the structures of the compound fulfilling the role of the “guest” may be different [11].
In turn, the chemical properties of starch, as well as other chemical compounds, are closely related to its reactivity. Starch is a polymer that chemically has most of the characteristics of alcohols, aldehydes, or ethers. The reactivity of native starch is relatively low and mainly relates to the hydroxyl groups of glucose units (substitution and oxidation reactions) as well as glycosidic bonds between individual polysaccharide mers (depolymerization reactions such as dextrinization). It mainly depends on factors such as the type of ring conformation, intermolecular bonds, electron density on oxygen atoms, and spherical reactions with neighboring chemical groups [18]. Among the reactions that starch may undergo, we can generally distinguish two groups, i.e., reactions that cause complete structural disintegration, including the breakdown of glycosidic bonds and granule degradation, and reactions resulting in a small, partial grain distribution, mainly due to the breakdown of intermolecular hydrogen bonds. Technically, due to the complicated granular structure of starch—and thus difficult access to hydroxyl groups and glycosidic bonds—mostly solvent reactions or the initial stage of starch gelatinization are used [19]. Homogeneous reactions, however, are not the only way to the modification of this polysaccharide. In the literature reports of effective solvent-free reactions, these are known to be carried out in a solid phase, heated conventionally or using a microwave radiation [20,21].In turn, the chemical properties of starch, as well as other chemical compounds, are closely related to its reactivity. Starch is a polymer that chemically has most of the characteristics of alcohols, aldehydes, or ethers. The reactivity of native starch is relatively low and mainly relates to the hydroxyl groups of glucose units (substitution and oxidation reactions) as well as glycosidic bonds between individual polysaccharide mers (depolymerization reactions such as dextrinization). It mainly depends on factors such as the type of ring conformation, intermolecular bonds, electron density on oxygen atoms, and spherical reactions with neighboring chemical groups [18]. Among the reactions that starch may undergo, we can generally distinguish two groups, i.e., reactions that cause complete structural disintegration, including the breakdown of glycosidic bonds and granule degradation, and reactions resulting in a small, partial grain distribution, mainly due to the breakdown of intermolecular hydrogen bonds. Technically, due to the complicated granular structure of starch—and thus difficult access to hydroxyl groups and glycosidic bonds—mostly solvent reactions or the initial stage of starch gelatinization are used [19]. Homogeneous reactions, however, are not the only way to the modification of this polysaccharide. In the literature reports of effective solvent-free reactions, these are known to be carried out in a solid phase, heated conventionally or using a microwave radiation [20][21].
3. The Methods to Improve Processing Properties of Starch
Natural starch does not exhibit a good processing property. Its high brittleness, lack of compatibility with hydrophobic polymers due to its highly hydrophilic nature, poor processing quality resulting from its high viscosity, low resistance to external factors during storage mainly moisture are its main disadvantages, constituting a significant limitation in the possibility of use in various industries. However, the unflagging interest in this polymer results from the fact that despite many disadvantages, it has a range of very significant advantages (
Figure 2). First of all, it is a natural, renewable, and biodegradable polymer, which is of great importance in the era of searching for environmentally friendly materials and technologies and reducing its pollution. Therefore, native starches undergo various types of modifications, usually to improve mechanical, processing, or utility properties [22]. They can be divided into four basic groups: physical, chemical, biochemical, and genetic modifications, also known as biotechnological. However, additional modification methods are mentioned more often in which more processes and reactions are used in parallel or in series. These are so-called dual and even mixed modifications [7]. The following are methods of starch modification toward non-food applications, mainly relevant to the packaging industry. The focus was on processes and processing increasing its plasticity, compatibility with natural and synthetic hydrophobic polymers, and increasing resistance to external factors, while maintaining the advantages of non-toxicity and biodegradability [22].

Advantages and disadvantages of starch as a potential packaging material.
References
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489.
- Williams, C.K.; Hillmyer, M.A. Polymers from renewable resources: A perspective for a special issue of polymer reviews. Polym. Rev. 2008, 48, 1–10.
- Kapusniak, J.; Jochym, K.; Bajer, K.; Bajer, D. Przegląd metod chemicznej modyfikacji skrobi. Przem. Chem. 2011, 90, 1521–1526.
- Bajer, K. Skrobia jako potencjalny surowiec materiałowy. Biotworzywa 2015, 1, 30–34.
- Chen, Q.; Yu, H.; Wang, L.; Abdin, Z.; Chen, Y.; Wang, J.; Zhou, W.; Yang, X.; Khan, R.U.; Zhang, H.; et al. Recent progress in chemical modification of starch and its applications. Rsc Adv. 2015, 5, 67459–67474.
- Chiu, C.; Solarek, D. Modification of Starches. In Starch: Chemistry and Technology; Academic Press: Cambridge, MA, USA, 2009; pp. 629–655.
- Gilet, A.; Quettier, C.; Wiatz, V.; Bricout, H.; Ferreira, M.; Rousseau, C.; Monfliera, E.; Tilloy, S. Unconventional media and technologies for starch etherification and esterification. Green Chem. 2018, 20, 1152–1168.
- Ptak, S.; Roczkowska, M.; Zarski, A.; Kapusniak, J. Esterification of starch with fatty acids. New opportunities and challenges. Przem. Chem. 2014, 4, 472–479.
- Stergiou, P.Y.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Hatziloukas, E.; Afendra, A.; Pandey, A.; Papamichael, E.M. Advances in lipase catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859.
- Van Rantwijk, F.; Madeira Lau, R.; Sheldon, R.A. Biocatalytic transformations in ionic liquids. Trends. Biotechnol. 2003, 21, 131–138.
- Tegge, G. Skrobia i jej Pochodne; PTTŻ Odział Małopolski: Kraków, Polska, 2010.
- Robyt, J.F. Starch: Structure, Properties, Chemistry and Enzymology. In Glycoscience; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1437–1472.
- Bertoft, E. Understanding Starch Structure: Recent Progress. Agronomy 2017, 7, 56.
- Ai, Y.; Jane, J. Understanding Starch Structure and Functionality. In Starch in Food: Structure, Function and Application; Woodhead Publishing: Cambridge, MA, USA, 2018; pp. 151–178.
- Available online: (accessed on 25 November 2020).
- Jane, J.; Chen, Y.Y.; Lee, L.F.; Mcpherson, A.E.; Wong, K.S.; Radosavljevic, M.; Kasemsuwan, T. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999, 76, 629–637.
- Hoover, R. Composition, molecular structure, and physicochemical properties of tuber and root starches: Review. Carbohyd. Polym. 2001, 45, 253–267.
- Tomasik, P.; Fiedorowicz, M.; Para, A. Novelties in chemical modification of starch. In Starch: Progress in Structural Studies, Modifications and Applications; Polish Society of Food Technologists: Kraków, Polska, 2004; pp. 301–355.
- Tomasik, P.; Schilling, C.H. Chemical modification of starch. Adv. Carbohyd. Chem. Bi. 2004, 59, 175–204.
- Kapusniak, J.; Siemion, P. Thermal reactions of starch with long-chain unsaturated fatty acids. Part 2. Linoleic acid. J. Food. Eng. 2007, 78, 323–332.
- Staroszczyk, H.; Tomasik, P.; Janas, P.; Poreda, A. Esterification of starch with sodium selenite and selenate. Carbohyd. Polym. 2007, 69, 299–304.
- Angellier, H.; Molina-Boisseau, S.; Dole, P.; Dufresne, A. Thermoplastic starch-waxy maize starch nanocrystals nanocomposites. Biomacromolecules 2006, 7, 531–539.