Leishmaniasis is a tropical and subtropical poverty-related disease caused by an intracellular parasite belonging to the genus Leishmania. Humans are generally infected via the bite of a sandfly, mostly Phlebotomus and Lutzomyia, around the world. According to reliable reports, 0.7–1 million new cases of the disease are notified annually, and 12–15 million people are now infected with the disease in different parts of the world.
- cutaneous leishmaniasis
- visceral leishmaniasis
- promastigote
- amastigote
- alternative medicine
- natural product
1. Introduction
Depending on the geographical distribution, various species of Leishmania such as L. tropica, L. major, L. donovani, L. infantum, L. mexicana, L. braziliensis, and L. amazonensis can cause different clinical forms of the disease [1][2]. Considering the classification and clinical picture of leishmaniasis in humans, the diseases are divided into four forms of cutaneous (CL), mucocutaneous (NCL), diffuse cutaneous (DCL), and visceral or kala-azar leishmaniasis (VL) [3][4].
There are a number of systemic and local therapeutic strategies for the treatment of various forms of leishmaniasis, including drugs (e.g., pentavalent antimony derivatives such as meglumine antimoniate (Glucantime®) and sodium stibogluconate (Pentostam®), miltefosine, pentamidine, amphotericin B (Amp B), and paromomycin), as well as physical treatments (e.g., cryotherapy, surgery, thermotherapy, and laser therapy) [5]. Based on recent studies, the current conventional chemotherapeutics generally have difficulty reaching the target tissues at the applied doses and are also linked to adverse side effects on healthy tissues [6], indicating that the drug delivery systems must improve the efficacy, tolerability, specificity, and therapeutic index of anti-leishmanial drugs. Moreover, unresponsiveness to these anti-leishmanial compounds, even to their higher doses, is regularly reported in some parts of the world [6][7]. These limitations motivate researchers to discover an effective alternative agent with low toxicity in natural compounds as a major source of medications with various therapeutics characteristics.
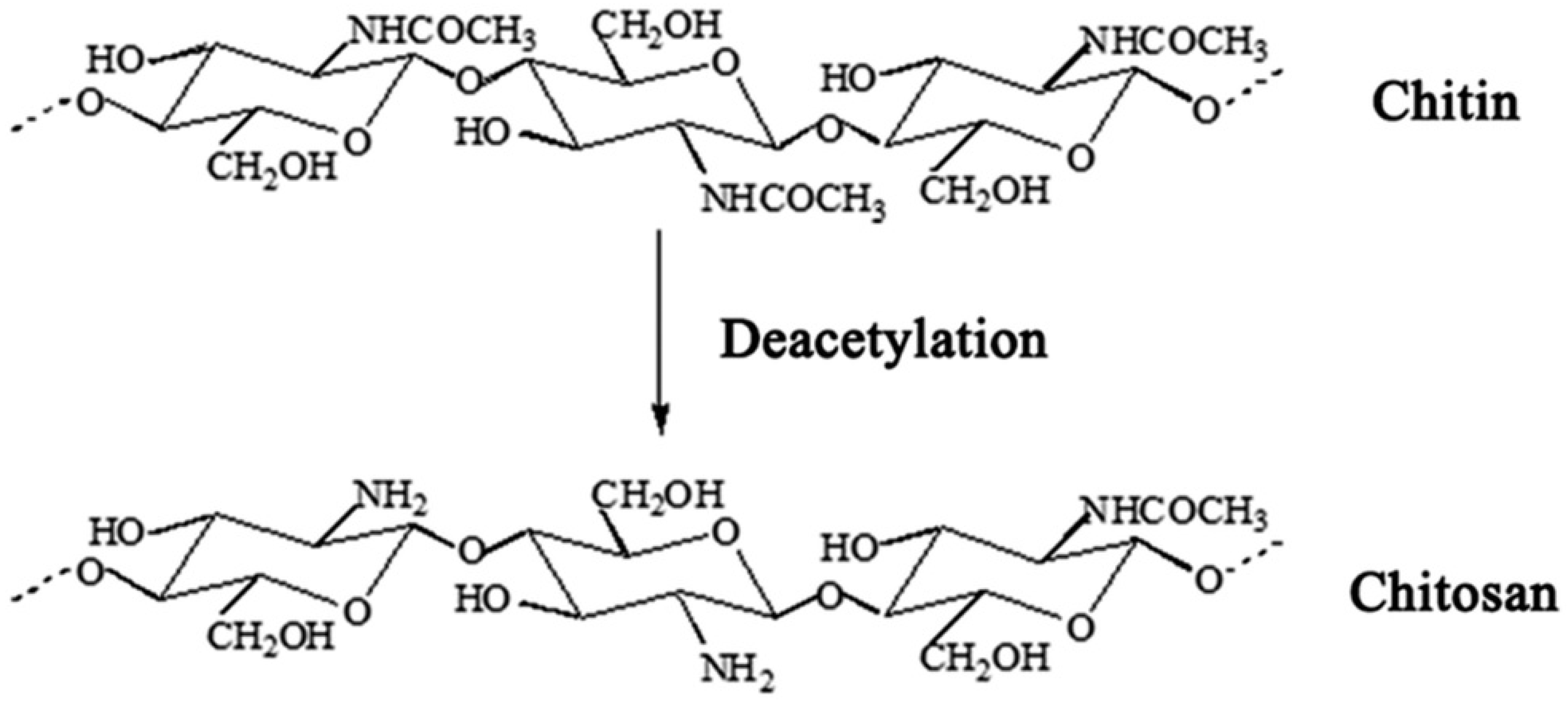
Chitosan (poly-(b-1/4)-2-amino-2-deoxy-D-glucopyranose) is the general name used for a group of natural polysaccharide polymers produced by deacetylation of chitin (Figure 1) [8][9][10]. In recent years, the use of chitosan and its derivatives has attracted the attention of many researchers in medical and pharmaceutical sciences [11] due to its unique properties such as potent biological properties, low toxicity, biocompatibility, biodegradability, immunomodulatory [12], and anti-cancer, anti-nociceptive, anti-oxidant, anti-inflammatory, and anti-microbial properties [13][14].
Recent studies have demonstrated that the preparation of chitosan-based biomedical drugs such as nanoparticles, hydrogels, coatings, suspensions, powders, membranes, and films can impact the pharmaceutical and biomedical effects of these agents [15][16]. Recently, the antimicrobial activities of chitosan and its derivatives have been reported against a wide range of pathogenic viruses, bacteria, filamentous and yeast-like fungi [17][18], and helminthic and protozoan parasites [19][20]. Considering the anti-parasitic properties of chitosan and its derivatives, several investigations have demonstrated their potent anti-parasitic effects against some pathogenic strains such as Cryptosporidium spp. [21], Echinococcus granulosus [22], Leishmania spp. [19][20], and Toxoplasma gondii [23][24].
2. Results and Discussion
Chitosan as a natural agent with diverse biological activities is generally found in the shells of crustaceans, such as crab, shrimp, squid pen, and crawfish; however, recent investigations have reported that chitosan can be produced from some fungi [11][12][13].
2.1. Treatments Using Chitosan as Vehicle
Nowadays, it has been demonstrated that chitosan, its derivatives, and chitosan-based nanomaterials are able to possibly remove barriers in the carrying of drugs, thus improving the efficacy of the drug and subsequently the targeted drug therapy [25]. The findings demonstrated that the most used synthetic drugs for combination therapy in vivo and in vitro were amphotericin B (14%, 70.0%), followed by miltefosine (3%, 15.0%) and doxorubicin hydrochloride (2%, 10.0%). Although most of the studies in this review use chitosan in combination with other drugs, however, chitosan and its derivatives without combination with common drugs have been considered in some studies.
Malli et al. (2019) have also demonstrated that the nanoparticles of poly (isobutyl-cyanoacrylate) coated with chitosan have potent anti-leishmanial effects on L. major promastigotes through morphological changes such as the aberrant shape and swelling of mitochondria and parasitic vacuoles [26]. In a study conducted by Feizabadi et al. (2019), it has been proven that chitosan combined with L. major secretory and excretory proteins can improve the ability of infected macrophages to remove parasites by decreasing apoptosis [27].
For example, Lima et al. [28] have reported that chitosan-silver nanoparticles have more anti-leishmanial activity than chitosan on L. amazonensis promastigotes with the IC50 values of 1.69 and 7.81 µg/mL, respectively. In the study conducted by Seyyed Tabaei et al. [29] have showed that chitosan-polyethylene oxide-berberine nanofibers has potent therapeutic effects on healing of CL induced by L. major in BALAB/C mice through reducing the parasite burden, decreasing the lesion size as well as change in the epidermis and dermis.
In recent years, the anti-parasitic activities of chitosan and its various derivatives/formulations have been studied against several parasitic pathogens such as C. pavum [21], Echinococcus spp. [22], and T. gondii [23][24]. For example, Mammeri et al. (2018) demonstrated that chitosan significantly decreased the viability of Cryptosporidium parvum oocysts by >95% after 24 h of treatment with chitosan mix (C-Mix) and chitosan N-acetyl-D-glucosamine (CNAD). They also reported that C-Mix (34.5%) and CNAD (56%) significantly decreased the oocysts’ shedding by 34.5% and 56% in newborn mice infected with cryptosporidiosis, respectively [21]. Torabi et al. (2018) have demonstrated that chitosan-praziquantel and chitosan-albendazole nanoparticles especially in combination at the doses of 1, 5, and 10 μg/mL significantly reduced the viability of microcysts, weight and number of cysts in vitro and in vivo [22]. In the study conducted by Teimouri et al. (2018), it has been proven that low molecular weight chitosan nanoparticles completely killed the tachyzoites at the concentration of 500 and 1000 ppm in vitro; they also showed that this compound considerably increased the survival time of infected mice with T. gondii RH strain from 6 to 8 days after infection [23].
2.2. Possible Antimicrobial Mechanisms of Chitosan
The precise antimicrobial mechanism of action of chitosan is yet to be fully understood; still, based on the literature, the most likely antimicrobial mechanisms of action of chitosan include the disruption of the cell wall and, consequently, an effect on the membrane’s permeability, inhibition of DNA replication, cell death, and bindings to the trace metal elements resulting in toxin production and microbial growth inhibition [30].
Mohammadi-Samani et al. (2011) have reported that chitosan nanoparticles containing Leishmania superoxide dismutase could be considered a nano-vaccine for leishmaniasis eradication by promoting the immune response toward cell-mediated immunity (TH1 cells producing IgG2a in mice) [31].
2.3. Cytotoxicity Effects of Chitosan
With respect to the cytotoxic effects of chitosan and its various formulations, Karam et al. (2020) found that chitosan nanocapsules containing the essential oil of Matricaria chamomilla have no significant cytotoxicity against macrophage cells with a CC50 (the 50% cytotoxic concentration) value of 207.92 ± 18.53 μg/mL compared to 19.71 ± 1.73 μg/mL for essential oil alone [32]. Another study conducted by Chaubey et al. (2018) indicated that the mannose-conjugated chitosan nanoparticles of curcumin had no significant cytotoxicity against the J774A.1 macrophage cell line with a CC50 value of 26 ± 0.60 mg/mL [33]. Recently, Esfandiari et al. (2019) have reported that paromomycin-loaded mannosylated chitosan nanoparticles had no considerable cytotoxicity against the human monocyte cell line of THP-1 cells with a CC50 value of 3911 μg/mL [34].
3. Conclusions
Studies in recent years revealed that chitosan, its derivatives, and chitosan-based nanomaterials are able possibly remove barriers in the carrying of drugs thus improving the efficacy of the drug and subsequently the targeted drug therapy. Based on the literature, various forms of drugs based on chitosan and their derivatives exhibited significant antileishmanial activity against various Leishmania spp, in vitro and in vivo. The results showed that chitosan and chitosan-based particles could be considered as an alternative and complementary source of valuable components against leishmaniasis with a high safety index. However, more studies are required to elucidate this finding, particularly in clinical settings.
References
- Hepburn, N.C. Cutaneous leishmaniasis: Clinical dermatology Review article. Clin. Exp. Dermatol. Clin. Dermatol. 2000, 25, 363–370.
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970.
- McGwire, B.S.; Satoskar, A.R. Leishmaniasis: Clinical syndromes and treatment. Qjm: Int. J. Med. 2014, 107, 7–14.
- Scorza, B.M.; Carvalho, E.M.; Wilson, M.E. Cutaneous Manifestations of Human and Murine Leishmaniasis. Int. J. Mol. Sci. 2017, 18, 1296.
- Bi, K.; Chen, Y.; Zhao, S.; Kuang, Y.; Wu, C.-H.J. Current Visceral Leishmaniasis Research: A Research Review to Inspire Future Study. BioMed Res. Int. 2018, 2018, 1–13.
- WHO Expert Committee on the Control of the Leishmaniases. Meeting, World Health Organization. In Proceedings of the Control of the Leishmaniases: Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, Switzerland, 22–26 March 2010; World Health Organization: Copenhagen, Denmark, 2010.
- Monzote, L. Current treatment of leishmaniasis: A review. Open Antimicrob. Agents J. 2009, 1, 9–19.
- Santos, D.O.; Coutinho, C.E.R.; Madeira, M.F.; Bottino, C.G.; Vieira, R.T.; Nascimento, S.B.; Bernardino, A.; Bourguignon, S.C.; Corte-Real, S.; Pinho, R.T.; et al. Leishmaniasis treatment—a challenge that remains: A review. Parasitol. Res. 2008, 103, 1–10.
- Oliveira, L.F.; Schubach, A.O.; Martins, M.M.; Passos, S.L.; Oliveira, R.V.; Marzochi, M.C.; Andrade, C.A. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta Trop. 2011, 118, 87–96.
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, T.M.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843.
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174.
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368.
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487.
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507.
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-Based (Nano)Materials for Novel Biomedical Applications. Molecules 2019, 24, 1960.
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776.
- Guan, G.; Azad, A.K.; Lin, Y.; Kim, S.W.; Tian, Y.; Liu, G.; Wang, H. Biological Effects and Applications of Chitosan and Chito-Oligosaccharides. Front. Physiol. 2019, 10, 516.
- Rozman, N.A.S.; Tong, W.Y.; Leong, C.R.; Tan, W.N.; Hasanolbasori, M.A.; Abdullah, S.Z. Potential Antimicrobial Applications of Chitosan Nanoparticles (ChNP). J. Microbiol. Biotechnol. 2019, 29, 1009–1013.
- Rabea, E.I.; Badawy, M.E.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465.
- Varshosaz, J.; Arbabi, B.; Pestehchian, N.; Saberi, S.; Delavari, M. Chitosan-titanium dioxide-glucantime nanoassemblies effects on promastigote and amastigote of Leishmania major. Int. J. Biol. Macromol. 2018, 107, 212–221.
- Mammeri, M.; Chevillot, A.; Thomas, M.; Polack, B.; Julien, C.; Marden, J.-P.; Auclair, E.; Vallée, I.; Adjou, K.T. Efficacy of chitosan, a natural polysaccharide, against Cryptosporidium parvum in vitro and in vivo in neonatal mice. Exp. Parasitol. 2018, 194, 1–8.
- Torabi, N.; Dobakhti, F.; Faghihzadeh, S.; Haniloo, A. In vitro and in vivo effects of chitosan-praziquantel and chitosan-albendazole nanoparticles on Echinococcus granulosus Metacestodes. Parasitol. Res. 2018, 117, 2015–2023.
- Teimouri, A.; Azami, S.J.; Keshavarz, H.; Esmaeili, F.; Alimi, R.; Mavi, S.A.; Shojaee, S. Anti-Toxoplasma activity of various molecular weights and concentrations of chitosan nanoparticles on tachyzoites of RH strain. Int. J. Nanomed. 2018, 13, 1341–1351.
- Cheraghipour, K.; Masoori, L.; Ezzatkhah, F.; Salimikia, I.; Amiri, S.; Makenali, A.S.; Taherpour, F.; Mahmoudvand, H. Effect of chitosan on Toxoplasma gondii infection: A systematic review. Parasite Epidemiol. Control. 2020, 11, e00189.
- Suman Gupta, N. Visceral leishmaniasis: Experimental models for drug discovery. Indian J. Med. Res. 2011, 133, 27–39.
- Malli, S.; Pomel, S.; Dennemont, I.; Loiseau, P.M.; Bouchemal, K. Combination of amphotericin B and chitosan platelets for the treatment of experimental cutaneous leishmaniasis: Histological and immunohistochemical examinations. J. Drug Deliv. Sci. Technol. 2019, 50, 34–41.
- Feizabadi, E.; Zavaran Hosseini, A.; Soudi Sara, K. Studying the role of chitosan nanoparticle loaded with Leishmania major Secretory and excretory antigens on the number of apoptotic macrophages in parasite sensitive mouse. Daneshvar Med. Basic Clin. Res. J. 2020, 26, 9–18.
- Lima, D.D.S.; Gullon, B.; Cardelle-Cobas, A.; Brito, L.M.; Rodrigues, K.A.F.; Quelemes, P.V.; Ramos-Jesus, J.; Arcanjo, D.D.R.; Plácido, A.; Batziou, K.; et al. Chitosan-based silver nanoparticles: A study of the antibacterial, antileishmanial and cytotoxic effects. J. Bioact. Compat. Polym. 2017, 32, 397–410.
- Seyyed Tabaei, S.J.; Rahimi, M.; Akbaribazm, M.; Ziai, S.A.; Sadri, M.; Shahrokhi, S.R.; Rezaei, M.S. Chitosan-based nano-scaffolds as antileishmanial wound dressing in BALB/c mice treatment: Characterization and design of tissue regeneration. Iran J. Basic Med. Sci. 2020, 23, 788–799.
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661.
- Mohammadi-Samani, S.; Bahraini, D.; Shokri, J.; Kamali-Sarvestani, E.; Baezegar-Jalali, M.; Samiei, A.; Danesh-Bahreini, M.A.; Barzegar-Jalali, M. Nanovaccine for leishmaniasis: Preparation of chitosan nanoparticles containing Leishmania superoxide dismutase and evaluation of its immunogenicity in BALB/c mice. Int. J. Nanomed. 2011, 6, 835–842.
- Karam, T.K.; Ortega, S.; Nakamura, T.U.; Auzély-Velty, R.; Nakamura, C.V. Development of chitosan nanocapsules containing essential oil of Matricaria chamomilla L. for the treatment of cutaneous leishmaniasis. Int. J. Biol. Macromol. 2020, 162, 199–208.
- Chaubey, P.; Mishra, B.; Mudavath, S.L.; Patel, R.R.; Chaurasia, S.; Sundar, S.; Suvarna, V.; Monteiro, M. Mannose-conjugated curcumin-chitosan nanoparticles: Efficacy and toxicity assessments against Leishmania donovani. Int. J. Biol. Macromol. 2018, 111, 109–120.
- Esfandiari, F.; Motazedian, M.H.; Asgari, Q.; Morowvat, M.H.; Molaei, M.; Heli, H. Paromomycin-loaded mannosylated chitosan nanoparticles: Synthesis, characterization and targeted drug delivery against leishmaniasis. Acta Trop. 2019, 197, 105072.
