Targeted Muscle Reinnervation (TMR) is considered to be an innovative and relevant surgical technique for improving the prosthetic control for people with different amputation levels of the limb. Indeed, TMR surgery makes it possible to obtain reinnervated areas that act as biological amplifiers of the motor control. On the technological side, a great deal of research has been conducted in order to evaluate various types of myoelectric prosthetic control strategies, whether direct control or pattern recognition-based control. In the literature, different control performance metrics, which have been evaluated on TMR subjects, have been introduced, but no accepted reference standard defines the better strategy for evaluating the prosthetic control. Indeed, the presence of several evaluation tests that are based on different metrics makes it difficult the definition of standard guidelines for comprehending the potentiality of the proposed control systems. Additionally, there is a lack of evidence about the comparison of different evaluation approaches or the presence of guidelines on the most suitable test to proceed for a TMR patients case study. This review aims at identifying these limitations by
examining the several studies in the literature on TMR subjects, with different amputation levels, and proposing a standard method for evaluating the control performance metrics.
- Targeted Muscle Reinnervation (TMR)
- upper limb amputee
- prosthesis
- prosthetic control
- multi-DoF control
- pattern recognition
1. Introduction
After an upper-extremity amputation, the employment of TMR allows for improving the functionality of myoelectric prostheses: the reinnervation of residual muscles creates additional myoelectric control sites available for obtaining the multi-DoF prosthetic control, without the need of switching between modalities available on the device [1]. In 1995, Kuiken examined muscle recovery and related changes in the motor unit population of “hyper-reinnervated” rats [2]. Only in 2004, the first TMR surgery was performed on one human subject with bilateral shoulder disarticulation amputation [3] Figure 1Figure 2.
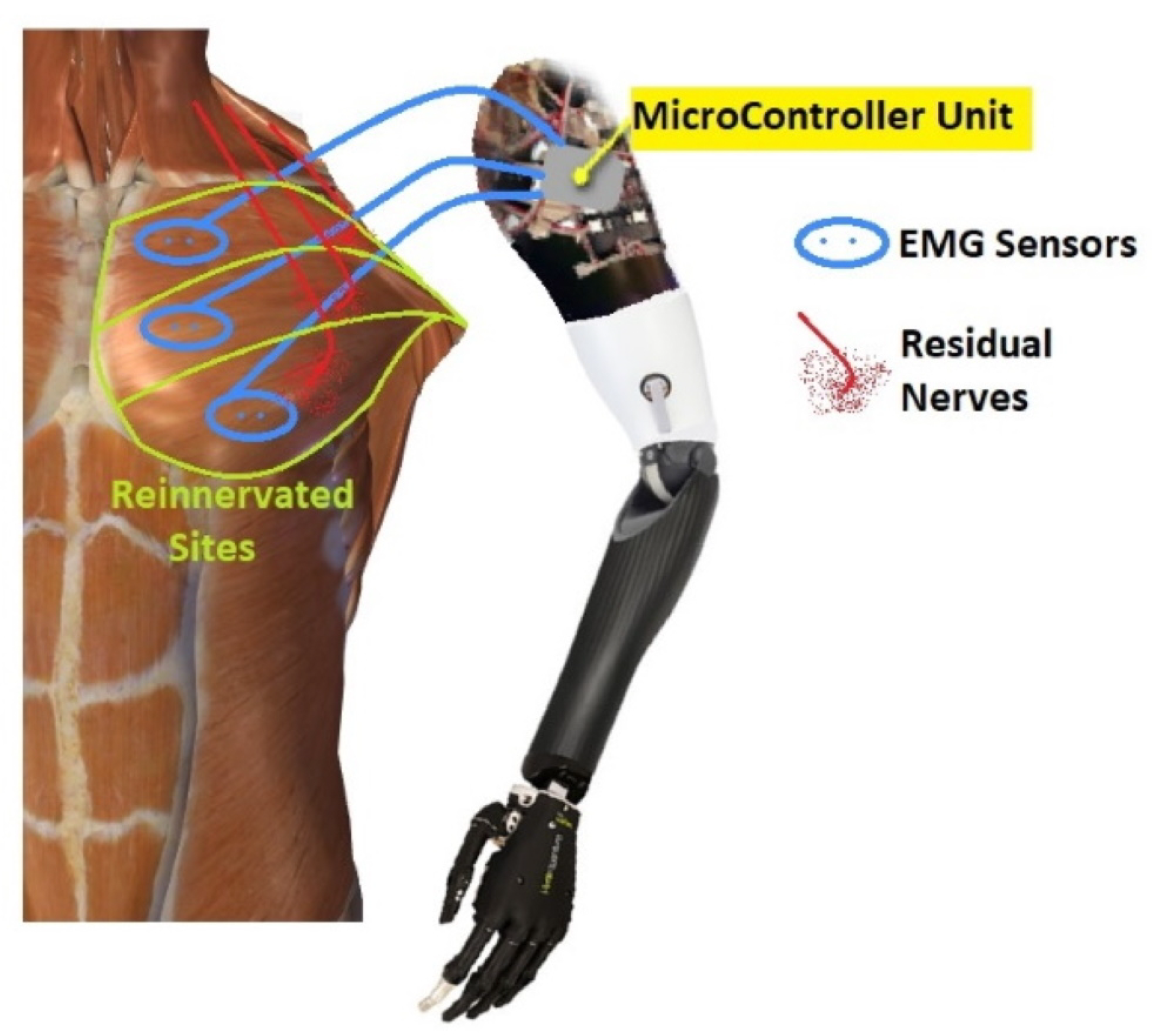
Figure 12.
In 2006, Kuiken introduced the following requirements to make TMR surgery successful: (i) separate regions of muscles and skin must be reinnervated by multiple donor nerves; (ii) EMG signals must be acquired from each target area; and, (iii) the prosthesis must be able to receive numerous EMG input signals and control several motors [4]. TMR can be performed for three different levels of amputation: shoulder disarticulation, transhumeral, and transradial amputation. The innervation strategies depend on the type of amputation [5]. For the shoulder disarticulated patients Figure 2Figure 3B, pectoralis muscles are usually denervated and then reinnervated with residual arm peripheral nerves [4]. Afterward, back muscles (if possible) are also reinnervated to have more active sites. For the transhumeral amputees Figure 2Figure 3A, the median nerve is transferred to the short head of the biceps motor branch to restore the function of hand closing or pronation; the ulnar nerve is transferred to a residual brachialis motor branch to have additional control sites for hand closing; finally, the radial nerve is reinnervated to the lateral head of the triceps motor branch in order to control hand opening or supination [6]. For transradial amputees, the control of multifunctional prosthetic hands can be reached by using additional Targeted Muscle Reinnervation signals for improving the function of intrinsic finger and thumb muscles: the distal median nerve is transferred to the flexor digitorum superficialis, while the ulnar nerve is reinnervated to the flexor carpi ulnaris [7]. When the muscles usually chosen cannot be reinnervated, as in [8], three bundles of the anterior tight muscle are used to obtain three active sites for the prosthetic control. The TMR is also an emerging technique for the treatment and reduction of the phantom limb pain (PLP) and neuroma pain [9], for the osseointegrated prostheses [10], and for the targeted sensory Reinnervation [11] of bidirectional neuroprosthetic devices. Finally, another important outcome is the use of TMR in the oncologic population, due to the potential to reduce pain without the use of opioids [12].
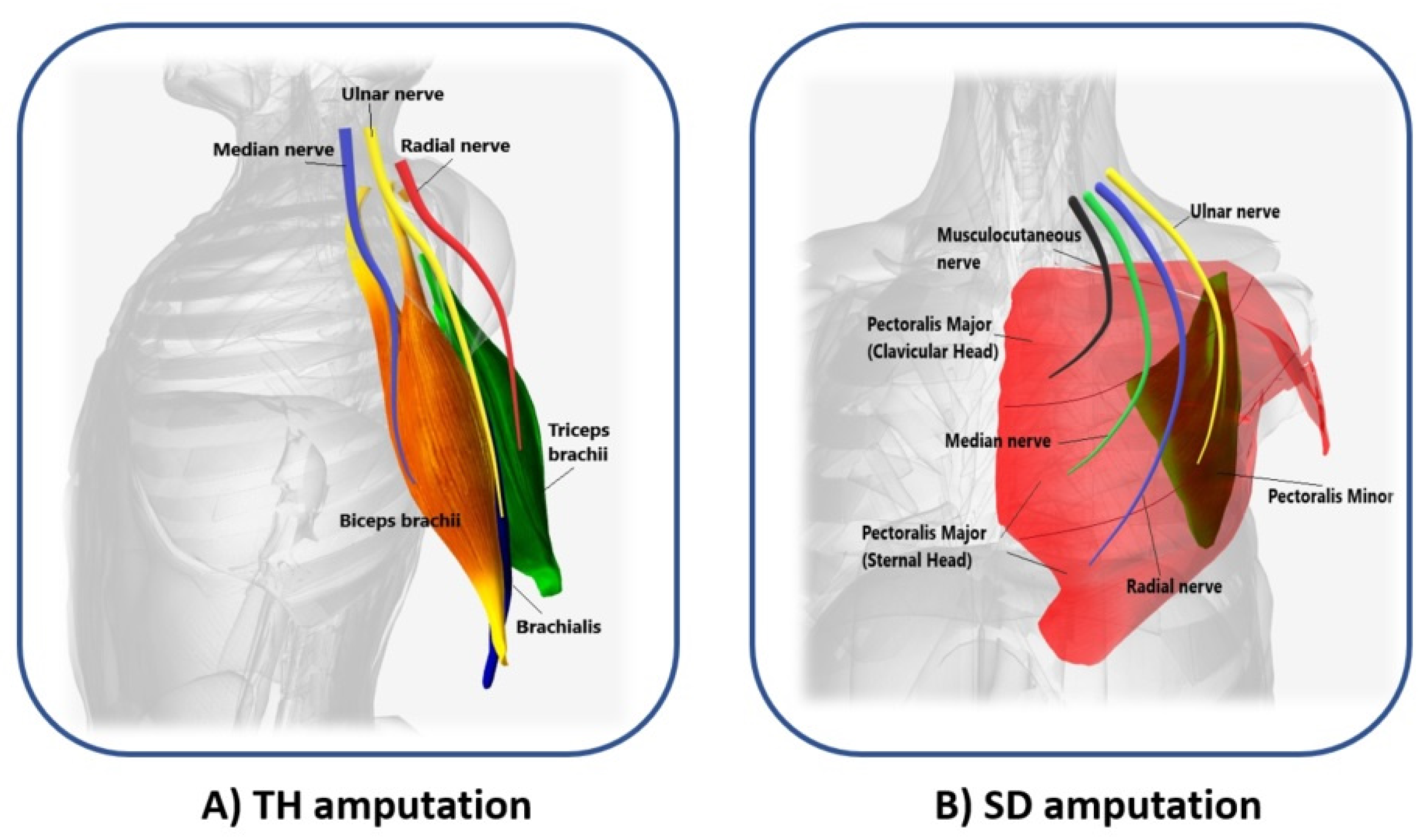
Figure 23. Scheme of the reinnervated sites for different levels of amputation. (A) Median (blue), ulnar (yellow), and radial (red) nerves transfer on biceps brachii (orange), brachialis (violet), and triceps brachii (green) muscles of transhumeral (TH) amputees; (B) Musculocutaneous (black), median (light green), radial (blue), and ulnar (yellow) nerves transfer on pectoralis major (clavicular and sternal head, in red), and pectoralis minor muscles (dark green) of shoulder disarticulation (SD) amputees.
2. Control Strategies
2.1. Direct Control
The control strategies where EMG signals are directly associated with a specific movement are named direct control strategies. Among them, the most used are on/off and proportional techniques. Multiples control techniques can be combined with the joint selection method to control multi-DoF prostheses. FigureFigure 5 3 shows the DC approach.
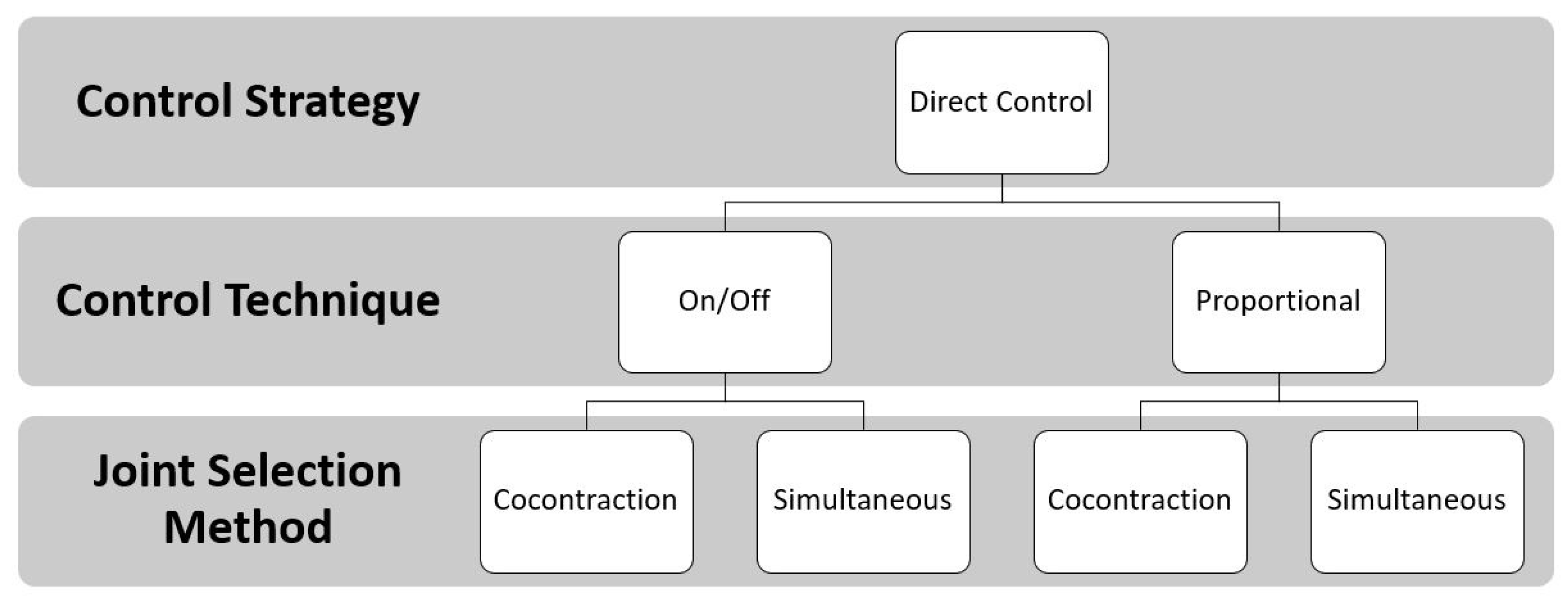
Figure 35. Direct Control approach: the EMG signals are the input to the controller unit. Two control techniques (the on/off and the proportional) defined the speed necessary to move the joint when the EMG signal is above a predefined threshold. The joint selection methods allow for the user to switch joints with muscle co-contraction or to select them simultaneously.
2.2. Control via Pattern Recognition
Generally, the pattern recognition strategies applied to the prosthetic control associated the several inputs based on sEMG signals of different movements to several outputs, as limb motions related to specific myoelectric patterns [13].
These PR algorithms consist of a first step that is based on feature extraction, in the time and frequency domain [14], to enhance information about EMG contraction in selected time windows. Subsequently, in the sequential control technique, a single classifier is trained that is based on linear or non-linear decision boundaries; instead, in the simultaneous control technique, multiple classifiers are trained to control multiple joints simultaneously or a single classifier is trained by considering discrete and combined movements as separate classes, as shown in Figure 4Figure 8.
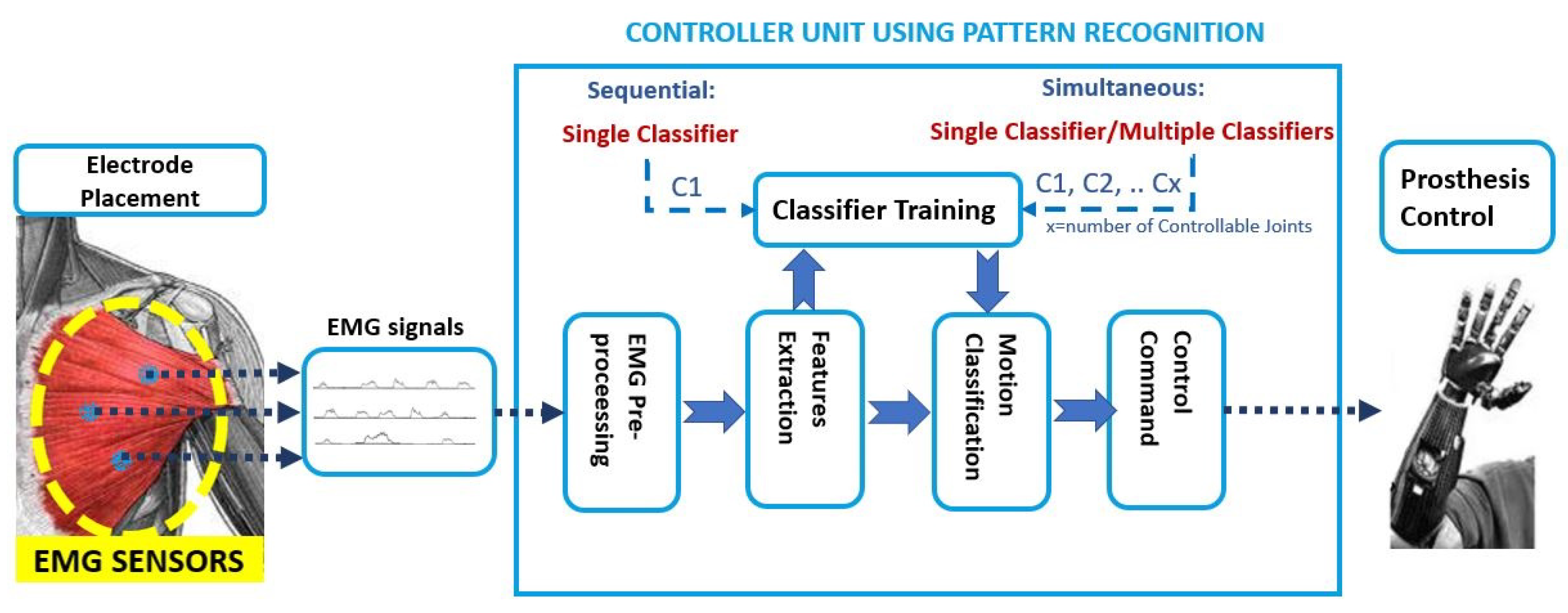
Figure 48. Pattern Recognition approach: the EMG signals are the input to the controller unit. Firstly the pre-processing step is done; then, in the features extraction step, the time and frequency domain features are used as input to train a single classifier or multiple classifiers. The classification output is the motion class to send as the command control to the prosthesis.
3. Conclusions
This entry further highlighted the presence of a variety of tests that were used for the functional performance evaluation, but there is a lack of standard criteria allowing to define which tests are the most suitable for the evaluation of prosthetic control for TMR patients with a different amputation level. In order to fill this gap, both the Box and Blocks and the Clothespin Relocation seem to be the most promising tests for evaluating the performance of the prosthetic systems.
References
- Myers, H.; Lu, D.; Gray, S.J.; Bruscino-Raiola, F. Targeted muscle reinnervation to improve electromyography signals for advanced myoelectric prosthetic limbs: A series of seven patients. ANZ J. Surg. 2020, 90, 591–596.
- Kuiken, T.A.; Childress, D.S.; Rymer, W.Z. The hyper-reinnervation of rat skeletal muscle. Brain Res. 1995, 676, 113–123.
- Kuiken, T.A.; Dumanian, G.A.; Lipschutz, R.D.; Miller, L.A.; Stubblefield, K. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet. Orthot. Int. 2004, 28, 245–253.
- Kuiken, T. Targeted reinnervation for improved prosthetic function. Phys. Med. Rehabil. Clin. 2006, 17, 1–13.
- Oh, C.; Carlsen, B.T. New innovations in targeted muscle reinnervation: A critical analysis review. JBJS Rev. 2019, 7, e3.
- Kuiken, T.A.; Miller, L.A.; Lipschutz, R.D.; Lock, B.A.; Stubblefield, K.; Marasco, P.D.; Zhou, P.; Dumanian, G.A. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: A case study. Lancet 2007, 369, 371–380.
- Kuiken, T.A.; Barlow, A.K.; Hargrove, L.; Dumanian, G.A. Targeted muscle reinnervation for the upper and lower extremity. Tech. Orthop. (Rockv. MD) 2017, 32, 109.
- Bueno Jr, R.A.; French, B.; Cooney, D.; Neumeister, M.W. Targeted muscle reinnervation of a muscle-free flap for improved prosthetic control in a shoulder amputee: Case report. J. Hand Surg. 2011, 36, 890–893.
- Souza, J.M.; Cheesborough, J.E.; Ko, J.H.; Cho, M.S.; Kuiken, T.A.; Dumanian, G.A. Targeted muscle reinnervation: A novel approach to postamputation neuroma pain. Clin. Orthop. Relat. Res. 2014, 472, 2984–2990.
- Mastinu, E.; Brånemark, R.; Aszmann, O.; Ortiz-Catalan, M. Myoelectric signals and pattern recognition from implanted electrodes in two TMR subjects with an osseointegrated communication interface. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 5174–5177.
- Hebert, J.S.; Chan, K.M.; Dawson, M.R. Cutaneous sensory outcomes from three transhumeral targeted reinnervation cases. Prosthet. Orthot. Int. 2016, 40, 303–310.
- Roubaud, M.S. Targeted Muscle Reinnervation in the Oncologic Population: A Literature Review and Current Practice. Curr. Surg. Rep. 2020, 8, 1–7.
- Ortiz-Catalan, M.; Håkansson, B.; Brånemark, R. Real-time and simultaneous control of artificial limbs based on pattern recognition algorithms. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 756–764.
- Tkach, D.; Huang, H.; Kuiken, T.A. Study of stability of time-domain features for electromyographic pattern recognition. J. Neuroeng. Rehabil. 2010, 7, 21.
- Tkach, D.C.; Young, A.J.; Smith, L.H.; Rouse, E.J.; Hargrove, L.J. Real-time and offline performance of pattern recognition myoelectric control using a generic electrode grid with targeted muscle reinnervation patients. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 727–734.
- Wurth, S.M.; Hargrove, L.J. A real-time comparison between direct control, sequential pattern recognition control and simultaneous pattern recognition control using a Fitts’ law style assessment procedure. J. Neuroeng. Rehabil. 2014, 11, 91.
- Young, A.J.; Smith, L.H.; Rouse, E.J.; Hargrove, L.J. A comparison of the real-time controllability of pattern recognition to conventional myoelectric control for discrete and simultaneous movements. J. Neuroeng. Rehabil. 2014, 11, 5.
- Young, A.J.; Smith, L.H.; Rouse, E.J.; Hargrove, L.J. A new hierarchical approach for simultaneous control of multi-joint powered prostheses. In Proceedings of the 2012 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24–27 June 2012; pp. 514–520.
- Young, A.J.; Smith, L.H.; Rouse, E.J.; Hargrove, L.J. Classification of simultaneous movements using surface EMG pattern recognition. IEEE Trans. Biomed. Eng. 2012, 60, 1250–1258.
