Graphene quantum dots (GQD) is an efficient nanomaterial composed of one or more layers of graphene with unique properties that combine both graphene and carbon dots (CDs). It can be synthesized using carbon-rich materials as precursors, such as graphite, macromolecules polysaccharides, and fullerene. This contribution emphasizes the utilization of GQD-based materials in the fields of sensing, bioimaging, energy storage, and corrosion inhibitors. Inspired by these numerous applications, various synthetic approaches have been developed to design and fabricate GQD, particularly bottom-up and top-down processes.
- Graphene Quantum Dots
1. Introduction
Carbon is an outstanding material and a more abundant element in the form of coal. It is considered as one of the world’s major sustainability matching with the green approach. The graphene shape has recently astonished the scientific community [1][2], as did the football fullerene shape, which was discovered with small needle-shaped carbon nanotubes (CNTs) in 1985 and characterized for the first time in 1991 [3][4]. These recent findings of unique carbon allotropes have given scientists, from all disciplines great interest and fascination. Zero-dimensional (0D), one-dimensional (1D), two-dimensional (2D), and three-dimensional (3D) graphite are included in the classification of carbon into graphite forms [5][6][7][8], as shown in
. Because of the van der Waals force between layers, 2D graphene is a single-layered nanomaterial that differs from multilayer graphite [9]. In the universe, the strongest and thinnest substance ever weighed is an atom-thick carbon material [10].
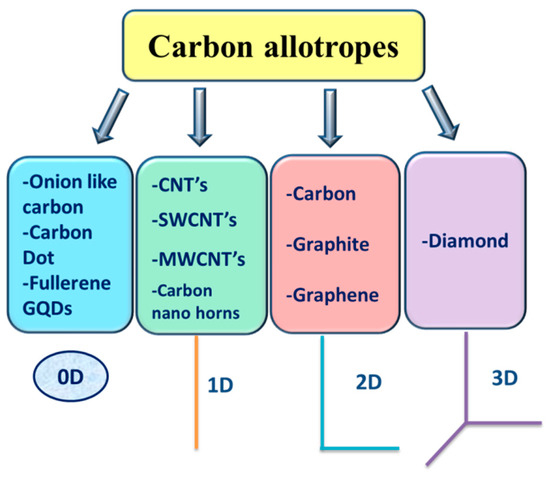
Carbon Materials Category.
Graphene was discovered at the University of Manchester in 2004 and since this time it is considered the marvelous substance of the 21st century [11]. The spectrum of materials bonded to graphene and graphene-based nanostructures are similar, but with different nomenclature, meaning that the carbon content contains one or more monolayers of graphene [12][13]. Furthermore, for the delicate handling and processing of graphene and its derivatives, many advanced techniques are now available. These techniques are used to manufacture items of various sizes from waste contents, such as C, O, H, or by manipulating surface groups, like hydroxyl, epoxy, carbonyl, and carboxyl [14][15]. In principle, there are four groups of different carbon materials, including carbon quantum dots (CQDs), carbon nanodots (CNDs), polymer carbon dots (CPDs), and graphene quantum dots (GQDs) with carbon dots (CDs) being used as a generic term [16]. Importantly, CDs families can be categorized based on their surface groups, properties, and basic structures of the carbon core. In this respect, CQDs have a crystal lattice accompanied by a spherical shape with surface chemical groups and also they have a quantum confinement effect (QCE) with luminescence features [17]. Moreover, it is possible to adjust the photoluminescence wavelength provided by the CQDs by changing its size [18]. Although GQDs have an apparent graphene lattice, they consist of one or more layers of fragments of graphene. Usually, the height of these GQDs is less than ten graphene layers with a transverse dimension of less than 100 nm. The surface groups in GQDs are adjacent to the defects or edges of the intermediate layers, impacting the different QCE and edge properties. QCE in GQDs are not only based on their size but also described in the planes of graphene by isolated conjugated π-domains, chemical groups, and a high carbonation degree [18]. QCE does not play a part in the properties of photoluminescence and is predominantly determined in the carbon core of graphite by the subdomain states and defect states. Carbon polymer dots (CPDs) are another class that contains a hybrid carbon/polymer structure in which the surface and center of the carbon are connected to a large number of functional/polymer classes [19][20][21]. Additionally, the key culprits for photoluminescence properties of CPDs are surface states, subdomain states, molecular states, and the crosslinking effect of radiation [22].
A comprehensive study of the assessment and classification of carbon dots has been reported based on properties and structure, with a particular focus on designing 0D GQDs in 2D lines [23][24]. This material form has many outstanding properties, such as good chemical inertia, excellent biocompatibility, high solubility parameter, fluorescent activity, photostability, emission of luminescence, long-term resistance to photobleaching, wide surface area, and better surface grafting [25][26]. In turn, these characteristics allow the study of new structural, optical, and electrical phenomena that are not present in other materials.
Over the past few years, a variety of carbon nanomaterials, such as high-surface-area, compatibility, prominent electron transport, excellent mechanical strength, and hydrophilicity have received extensive interest with graphene and graphene oxide (GO). The existence on its surface of different functional oxygen groups (carbonyl and carboxylate) which make GO with high hydrophilicity. The GO nanosheets have a high surface area that may also be an effective place to decorate and disperse other inorganic nanoparticles such as TiO
; in a recent study, reduced graphene oxide/TiO
nanocomposite was used for modification of thin-film nanocomposite reverse osmosis membranes. The rGO/TiO
nanocomposite, as a hydrophilic additive, was synthesized using a facile hydrothermal method the result demonstrates that The membrane containing 0.02 wt. % rGO/TiO
nanocomposite observed excellent RO performance involving 51.3 L/m
h flow of water, 99.45% NaCl rejection and exceptional chlorine good resistance [27][28]. Furthermore, graphene oxide sheet has also used with Ce ions in the photocatalytic efficiency of magnetite for photodegradation of oxytetracycline [29].
These unique features make this structural carbon category a promising candidate for different utilizations, such as biosensors, energy storage, bioimaging, and in redox electrochemical reactions by modified graphene surface of the TX-100 to understand its Electrochemistry interface [30][31][32].
GQDs demonstrate premium solubility in inorganic solvents such as THF, DMF, and further acetone. Further, DMSO and ethanol are commonly used solvents that provide good solubility. However, the applications of GQDs in bioimaging and selective drug delivery systems have been greatly affected by the increased solubility in aqueous solvents [33]. Moreover, the potential to be water-soluble is due to the units containing hydroxyl and carboxyl attached to the GQDs edges since the hydrophilicity of GQDs is enhanced by surface functionality, and it can be controlled by synthetic methods and hence, tuning the chemical properties [34].
Highly reliant upon the technique of GQDs synthesis was obtained, research is at a relatively early stage, considering the many superior advantages and properties; hence, many of the shortcomings of GQDs have yet to be overcome. Although many significant advantages and promising applications are possible, more research is required to enhance material properties and resolve some constraints. Moreover, electrical behavior resulting from the small size of GQD exhibits a quantum size effect. In addition, several obstacles must be met to take advantage of these specific properties [35]. The synthetic methodology of GQDs by the chemical route results in a remarkable heterogeneity in the size and the functionality of the surface. With such a broad variety of chemical features and measurements, the function of its particular properties is difficult to study [36]. Moreover, GQD’s photoluminescence and quantum confinement characteristics are highly dependent on size [37].
Various methods of synthesis can account for the major difference in chemical structure and scale. Thus, it is important to recognize the connection between dimensional variation depending on the synthesis process and optical properties in terms of applied and fundamental perspectives [38][39]. A fascinating report of the GQDs synthetic process was primarily evaluated on the size-dependent photoluminescence properties and the quantum size effect of GQDs [40]. Furthermore, because of their superior properties, the benchmark shows continuous improvement in functionalized and critical GQDs applications [41].
Carbohydrates are one of the most diverse and important classes of biomacromolecules in nature and provide well-defined chiral scaffolds ready for modification of the anomeric position and functionality of alcohol. Therefore, the use of carbohydrates as a starting material for GQDs synthesis is extremely attractive not only for their large quantity, availability, and heterogeneity, but also for their high-water solubility, low carbonization temperatures, low cost, and lack of toxicity. Not surprisingly, with all of these tuning options for GQDs synthesis, researchers have already begun to see the benefits of carbohydrates when they consider synthesizing new GQDs with improved properties. For example, simple monosaccharides such as glucose, glucosamine, mannose, fructose, and their common derivatives and disaccharides, such as sucrose, lactose, and maltose, have been used to create GQDs by various methods. Likewise, important natural biopolymers based on carbohydrates such as cellulose, dextran, β-cyclodextrin, chitin, chitosan, and hyaluronic acid, which vary not only in their basic composition but also in their physical and chemical properties which have been successfully used to obtain GQDs [42][43][44].
Polycyclic aromatic hydrocarbons (PAHs) are an organic hydrocarbon with two or more fused benzene rings. PAHs are aromatic compounds mainly produced by the natural manner and they are mostly toxic. The significant effort to degrade and track potentially such dangerous substances is desperately needed [45]. So, by using commercially available PAHs as precursors, we present a simple and efficient approach to PL GQDs based on the bottom-up approach. The PL GQDs obtained have 5–10 nm sizes and 0.5–2 nm thicknesses and shows better solubility of water and tunable fluorescence. We also show that, because of their stable fluorescence and low toxicity, the PL GQDs are not only promising for bioimaging but also effective for Fe
and hydrogen peroxide sensing [46].
In this review, in terms of size, convexity, surface, and solvent compliance, the methods currently proposed for the functionalization of GQDs and their properties were examined. Furthermore, it addressed pristine and modified GQDs applications in sensors, energy storage,
2. Synthetic Routes of GQDs
The primary and key method before using a material for a specific application is material synthesis. The variance in application outcomes usually depends on the morphology and material properties, which are primarily determined by the method of synthesis [47]. GQDs consisting of carbon-rich materials used as precursors, such as graphite, polysaccharides, fullerene, graphene oxide (GO), CNT, and carbon fiber (CF) [48][49]. To synthesize GQDs, there are two main methods used, namely, top-down and bottom-up strategies. These two techniques are difficult to synthesis the quantum dots in traditional semiconductors. Subsequently, as shown in
, carbonization or controlled synthesis techniques have been implemented to produce GQDs from acceptable organic molecules or polymers [50][51]. Furthermore, the different between top-down and bottom-up approaches were illustrated in
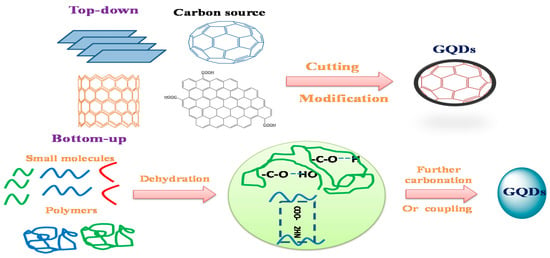
Two techniques for the production of fluorescence graphene quantum dots (GQDs): “top-down” breaking from complex particles and “bottom-up” from small molecules.
Comparison between top-down and bottom-up approaches.
| Basis for Comparison | Top-Down Approach | Bottom-Up Approach | ||||
|---|---|---|---|---|---|---|
| Basic | Successive cutting or grinding of bulk material to get nanoparticles | The buildup of material from bottom: atom or molecule to get nanoparticles | ||||
| Starting materials | Solid-state | The starting material is either gaseous or liquid | ||||
| Processing method | Physical method | Physical and chemical methods | ||||
| Advantages |
|
| ||||
| Disadvantages |
|
|
To obtain broad GQDs, controlled synthesis is precise but complex and involves several processing steps. However, tiny molecules or polymers suitable as GQDs are obtained by dehydration or combination when using the carbonation technique [52]. Sometimes, these procedures are out of balance, resulting in non-uniform GQDs proportions. Fortunately, due to the use of non-toxic reagents, GQDs are biocompatible. Further, as far as we know, the degradation of the carbonaceous material includes much of the top-down synthesis process. However, in terms of low efficiency, unforeseen structural damage, and heterogeneous morphology, these approaches have major drawbacks [53].
Oxidative degradation, hydrothermal/solvothermal processes, microwave/ ultrasonic processes, electrochemical oxidation, and chemical vapor deposition (CVD), pulsed laser ablation (PLA) are the most important top-down approaches mentioned [54][55][56]. The bottom-up methods, on the other hand, provide a controlled synthesis and provide good carbonation with a reasonable size range, high brightness, and satisfactory properties of the synthesized GQDs [36][51].
Recently, the effective use of starch as an innovative material for the synthesis of GQDs. Biocompatibility and imaging potential of synthesized GQDs were evaluated using MTT assay and CaSki cell lines, respectively [57]. Further, GQDs can be synthesized from fructose as a precursor in which hydrochloric acid and ethylenediamine were found to be strong chlorine and nitrogen donors for chlorine and nitrogen co-doped GQDs, respectively, which showed excellent stability [58].
On the other hand, the carbonization of sugar resulted in the breakdown of glycosidic linkages via dehydrogenation into elemental carbon. In this study, when subjected to serial MW heating with hydrothermal treatment glucose pyrolyzed to form GQDs and the control on GQDs size can be achieved by MW heating time [59].
In another work, investigated an effective utilization of the potential industrial by-product such as sugarcane molasses (SMs) for single-crystalline sulfur-doped (S-GQDs) synthesis via the hydrothermal method, the remarkable property of S-GQDs demonstrated by labeling the cytoplasmic area of HepG2 cells in-vitro with minimum uptake by normal DF-1 and HEK 293 cells [60]. Furthermore, the emulsion-template carbonization (E-TC) method has been used for the synthesis of GQDs by using honey and n-butanol water in oil emulsion by simple heating offering a quantum yield (QY) of about 3.6% [61].
In another example, efficient synthesis of GQDs using rice grains as a carbon source. Heating of starch powder has resulted in the formation of glucose oligomers, further heating of these oligomers offers nucleation and pyrolysis resulted in black carbonaceous powder comprising GQDs [62].
In a recent study it was designed a fluorescence-responsive sodium hexametaphosphate sensor depend on a reduced graphene quantum dot/chitosan formula for ALP, the bright blue emissions rGQDs with high negative charged hydroxyl group was prepared using NaBH
. In the production of the probe for ALP detected was fabricated by the combination of rGQD and chitosan via auto assembly. The chitosan charged biopolymer simultaneously displays the transformation-induced fluorescence quenching of rGQDs as well as electrostatic appeal. The method established shows good ALP selectivity and has promising results when applied to real test samples. This low-
References
- Jacob-Lopes, E.; Santos, A.B.; Severo, I.A.; Deprá, M.C.; Maroneze, M.M.; Zepka, L.Q. Dual production of bioenergy in heterotrophic cultures of cyanobacteria: Process performance, carbon balance, biofuel quality and sustainability metrics. Biomass Bioenergy 2020, 142, 105756.
- Mudusu, D.; Reddy, N.K.; Lee, S.; Hahn, Y.-B. Recent advances in graphene monolayers growth and their biological applications: A review. Adv. Colloid Interface Sci. 2020, 283, 102225.
- Rajani, D.M.; Crasta, F.; Kottur, V.K.N. A Review on Carbon Nanotubes as Novel Drug Carriers in Cancer Therapy. In Proceedings of International Conference on Intelligent Manufacturing and Automation; Springer: Berlin/Heidelberg, Germany, 2020; pp. 459–467.
- Chen, L.; Hou, Z.; Liu, Y.; Luan, C.; Zhu, L.; Li, W. High strength and high ductility copper matrix composite reinforced by graded distribution of carbon nanotubes. Compos. Part A Appl. Sci. Manuf. 2020, 138, 106063.
- Stieber, S.; Schröter, N.; Schiendorfer, A.; Hoffmann, A.; Reif, W. FlowFrontNet: Improving Carbon Composite Manufacturing with CNNs; University of Augsburg: Augsburg, Germany, 2020.
- Habert, G.; Röck, M.; Steininger, K.; Lupísek, A.; Birgisdottir, H.; Desing, H.; Chandrakumar, C.; Pittau, F.; Passer, A.; Rovers, R.; et al. Carbon budgets for buildings: Harmonising temporal, spatial and sectoral dimensions. Build. Cities 2020, 1, 429–452.
- Guo, F.; Yang, H.; Liu, L.; Han, Y.; Al-Enizi, A.M.; Nafady, A.; Kruger, P.E.; Telfer, S.G.; Ma, S. Hollow capsules of doped carbon incorporating sulfide and oxide core–shell nanoparticles derived from metal–organic framework composites for efficient oxygen electrocatalysis. J. Mater. Chem. A 2019, 7, 3624–3631.
- Rana, U.A.; Anis, A.; Nafady, A.; Al-Zahrani, S.M. Nitrogen and Phosphorus Co-Doped Crystalline Carbon Materials. U.S. Patent 10010866, 3 July 2018.
- Azadmanjiri, J.; Srivastava, V.K.; Kumar, P.; Sofer, Z.; Min, J.; Gong, J. Graphene-Supported 2D transition metal dichalcogenide van der waals heterostructures. Appl. Mater. Today 2020, 19, 100600.
- Rajesh, J. Flexible Material in Solar Panel for Potential Efficiency; Elsevier BV: Amsterdam, The Netherlands, 2020; Volume 33, pp. 1116–1120.
- Adetayo, A.; Runsewe, D. Synthesis and Fabrication of Graphene and Graphene Oxide: A Review. Open J. Compos. Mater. 2019, 9, 207–229.
- Tabandeh-Khorshid, M.; Kumar, A.; Omrani, E.; Kim, C.; Rohatgi, P. Synthesis, characterization, and properties of graphene reinforced metal-matrix nanocomposites. Compos. Part B Eng. 2020, 183, 107664.
- Gangu, K.K.; Maddila, S.; Mukkamala, S.B.; Jonnalagadda, S.B. Characteristics of MOF, MWCNT and graphene containing materials for hydrogen storage: A review. J. Energy Chem. 2019, 30, 132–144.
- Mohan, V.B. Handling and Risk Mitigation of Nanoscale Graphene and Related Materials: Some Considerations and Recommendations. C—J. Carbon Res. 2019, 5, 36.
- Sengupta, J.; Hussain, C.M. Graphene and its derivatives for Analytical Lab on Chip platforms. TrAC Trends Anal. Chem. 2019, 114, 326–337.
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316.
- Huang, J.; Li, L.; Chen, J.; Ma, F.; Yu, Y. Broad spectrum response flower spherical-like composites CdIn2S4/CdS modified by CQDs with up-conversion property for photocatalytic degradation and water splitting. Int. J. Hydrogen Energy 2020, 45, 1822–1836.
- Yeh, T.-F.; Huang, W.-L.; Chung, C.-J.; Chiang, I.-T.; Chen, L.-C.; Chang, H.-Y.; Su, W.-C.; Cheng, C.; Chen, S.-J.; Teng, H. Elucidating Quantum Confinement in Graphene Oxide Dots Based On Excitation-Wavelength-Independent Photoluminescence. J. Phys. Chem. Lett. 2016, 7, 2087–2092.
- Yu, Y.; Tang, P.; Barnych, B.; Zhao, C.; Sun, G.; Ge, M. Design and Synthesis of Core–Shell Carbon Polymer Dots with Highly Stable Fluorescence in Polymeric Materials. ACS Appl. Nano Mater. 2019, 2, 6503–6512.
- Teaima, M.H.; ElAsaly, M.K.; Omar, S.A.; El-Nabarawi, M.A.; Shoueir, K.R. Eco-friendly synthesis of functionalized chitosan-based nanoantibiotic system for potential delivery of linezolid as antimicrobial agents. Saudi Pharm. J. 2020, 28, 859–868.
- Shoueir, K.; Wassel, A.R.; Ahmed, M.K.; El-Naggar, M.E. Encapsulation of extremely stable polyaniline onto Bio-MOF: Photo-activated antibacterial and depletion of ciprofloxacin from aqueous solutions. J. Photochem. Photobiol. A Chem. 2020, 400, 112703.
- Goetz, K.P.; Taylor, A.D.; Paulus, F.; Vaynzof, Y. Shining Light on the Photoluminescence Properties of Metal Halide Perovskites. Adv. Funct. Mater. 2020, 30, 1910004.
- Zhou, X.; Wang, Y.; Gong, C.; Liu, B.; Wei, G. Production, structural design, functional control, and broad applications of carbon nanofiber-based nanomaterials: A comprehensive review. Chem. Eng. J. 2020, 402, 126189.
- Cirtoaje, C.; Petrescu, E.; Stan, C.; Rogachev, A. Electric Freedericksz transition in nematic liquid crystals with graphene quantum dot mixture. Appl. Surf. Sci. 2019, 487, 1301–1306.
- Nguyen, D.A.; Oh, H.M.; Duong, N.T.; Bang, S.H.; Yoon, S.J.; Jeong, M.S. Highly Enhanced Photoresponsivity of a Monolayer WSe2 Photodetector with Nitrogen-Doped Graphene Quantum Dots. ACS Appl. Mater. Interfaces 2018, 10, 10322–10329.
- Haque, E.; Kim, J.; Malgras, V.; Reddy, K.R.; Ward, A.; You, J.; Bando, Y.; Hossain, S.A.; Yamauchi, Y. Recent Advances in Graphene Quantum Dots: Synthesis, Properties, and Applications. Small Methods 2018, 2, 1800050.
- Safarpour, M.; Khataee, A.; Vatanpour, V. Thin film nanocomposite reverse osmosis membrane modified by reduced graphene oxide/TiO2 with improved desalination performance. J. Membr. Sci. 2015, 489, 43–54.
- Maio, A.; Scaffaro, R.; Riccobono, A.; Pibiri, I. Functionalization of Graphene with Molecules and/or Nanoparticles for Advanced Applications. In Handbook of Graphene; Archivio Istituzionale della Ricerca dell’Università degli Studi di Palermo Prodotti della Ricerca; Scrivener Publishing: Austin, TX, USA, 2019; Volume 1, pp. 559–609.
- Hassandoost, R.; Pouran, S.R.; Khataee, A.; Orooji, Y.; Joo, S.W. Hierarchically structured ternary heterojunctions based on Ce3+/ Ce4+ modified Fe3O4 nanoparticles anchored onto graphene oxide sheets as magnetic visible-light-active photocatalysts for decontamination of oxytetracycline. J. Hazard. Mater. 2019, 376, 200–211.
- Faridbod, F.; Sanati, A.L. Graphene Quantum Dots in Electrochemical Sensors/Biosensors. Curr. Anal. Chem. 2019, 15, 103–123.
- Gu, H.; Tang, H.; Xiong, P.; Zhou, Z. Biomarkers-based Biosensing and Bioimaging with Graphene for Cancer Diagnosis. Nanomaterials 2019, 9, 130.
- Jayaprakash, G.K.; Flores-Moreno, R. Quantum chemical study of Triton X-100 modified graphene surface. Electrochim. Acta 2017, 248, 225–231.
- Badıllıa, U.; Mollarasouli, F.; Bakirhan, N.K.; Ozkane, Y.; Özkan, S.A. Role of quantum dots in pharmaceutical and biomedical analysis, and its application in drug delivery. TrAC Trends Anal. Chem. 2020, 131, 116013.
- Bokare, A.; Nordlund, D.; Melendrez, C.; Robinson, R.; Keles, O.; Wolcott, A.; Erogbogbo, F. Surface functionality and formation mechanisms of carbon and graphene quantum dots. Diam. Relat. Mater. 2020, 110, 108101.
- Yan, Y.; Gong, J.; Chen, J.; Zeng, Z.; Huang, W.; Pu, K.; Liu, J.; Chen, P. Recent Advances on Graphene Quantum Dots: From Chemistry and Physics to Applications. Adv. Mater. 2019, 31, e1808283.
- Dervishi, E.; Ji, Z.; Htoon, H.; Sykora, M.; Doorn, S.K. Raman spectroscopy of bottom-up synthesized graphene quantum dots: Size and structure dependence. Nanoscale 2019, 11, 16571–16581.
- Nejad, S.S.; Babaie, A.; Bagheri, M.; Rezaei, M.; Abbasi, F.; Shomali, A. Effects of graphene quantum dot (GQD) on photoluminescence, mechanical, thermal and shape memory properties of thermoplastic polyurethane nanocomposites. Polym. Adv. Technol. 2020.
- Al-Ahmed, Z.A.; Al-Radadi, N.S.; Ahmedef, M.K.; Shoueir, K.; El-Kemary, M. Dye removal, antibacterial properties, and morphological behavior of hydroxyapatite doped with Pd ions. Arab. J. Chem. 2020, 13, 8626–8637.
- Elsayed, M.T.; Hassanbc, A.A.; Abdelaal, S.A.; Taher, M.M.; Ahmed, M.; Shoueir, K.R. Morphological, antibacterial, and cell attachment of cellulose acetate nanofibers containing modified hydroxyapatite for wound healing utilizations. J. Mater. Res. Technol. 2020, 9, 13927–13936.
- Temerov, F.; Belyaev, A.; Ankudze, B.; Pakkanen, T.T.; Beliaev, A. Preparation and photoluminescence properties of graphene quantum dots by decomposition of graphene-encapsulated metal nanoparticles derived from Kraft lignin and transition metal salts. J. Lumin. 2019, 206, 403–411.
- Wang, R.; Fan, H.; Jiang, W.; Ni, G.; Qu, S. Amino-functionalized graphene quantum dots prepared using high-softening point asphalt and their application in Fe3+ detection. Appl. Surf. Sci. 2019, 467, 446–455.
- Esmaeilzadeh, M.; Sadjadi, S.; Salehi, Z. Pd immobilized on hybrid of magnetic graphene quantum dots and cyclodextrin decorated chitosan: An efficient hydrogenation catalyst. Int. J. Biol. Macromol. 2020, 150, 441–448.
- Shende, P.; Pathan, N. Potential of carbohydrate-conjugated graphene assemblies in biomedical applications. Carbohydr. Polym. 2021, 255, 117385.
- Bayoumy, A.M.; Refaat, A.; Yahia, I.; Zahran, H.Y.; Elhaes, H.; Ibrahim, M.A.; Shkir, M. Functionalization of graphene quantum dots (GQDs) with chitosan biopolymer for biophysical applications. Opt. Quantum Electron. 2019, 52, 16.
- Sahoo, B.M.; Kumar, B.V.V.R.; Banik, B.K.; Borah, P. Polyaromatic Hydrocarbons (PAHs): Structures, Synthesis and their Biological Profile. Curr. Org. Synth. 2020, 17, 625–640.
- Zhou, L.; Geng, J.; Liu, B. Graphene Quantum Dots from Polycyclic Aromatic Hydrocarbon for Bioimaging and Sensing of Fe3+ and Hydrogen Peroxide. Part. Part. Syst. Charact. 2013, 30, 1086–1092.
- Xu, Y.; Shi, Z.; Shi, X.; Kai, Z.; Zhang, H. Recent progress in black phosphorus and black-phosphorus-analogue materials: Properties, synthesis and applications. Nanoscale 2019, 11, 14491–14527.
- Kaciulis, S.; Mezzi, A.; Soltani, P.; Pizzoferrato, R.; Ciotta, E.; Prosposito, P. Graphene quantum dots obtained by unfolding fullerene. Thin Solid Films 2019, 673, 19–25.
- Shen, S.; Wang, J.; Wu, Z.; Du, Z.; Tang, Z.; Yang, J. Graphene Quantum Dots with High Yield and High Quality Synthesized from Low Cost Precursor of Aphanitic Graphite. Nanomaterials 2020, 10, 375.
- Naumov, A.V.; Hasan, T.; Campbell, E.; Lin, C.-W.; Belcher, A.M. (Invited) In Vitro and In Vivo Near-Infrared Imaging with Biocompatible Bottom-up and Top-Down-Synthesized Graphene Quantum Dots. ECS Meet. Abstr. 2020, 648.
- Huang, D.; Zhou, H.; Wu, Y.; Wang, T.; Sun, L.; Gao, P.; Sun, Y.; Huang, H.; Zhou, G.; Hu, J. Bottom-up synthesis and structural design strategy for graphene quantum dots with tunable emission to the near infrared region. Carbon 2019, 142, 673–684.
- Raeyani, D.; Shojaei, S.; Kandjani, S.A.; Wlodarski, W. Synthesizing Graphene Quantum Dots for Gas Sensing Applications. Procedia Eng. 2016, 168, 1312–1316.
- Das, P.; Ganguly, S.; Banerjee, S.; Das, N.C. Graphene based emergent nanolights: A short review on the synthesis, properties and application. Res. Chem. Intermed. 2019, 45, 3823–3853.
- León, I.N.-D.; Johny, J.; Vázquez-Rodríguez, S.; García-Gómez, N.; Carranza-Bernal, S.; Mendivil, I.; Shaji, S.; Sepulveda-Guzman, S. Tuning the luminescence of nitrogen-doped graphene quantum dots synthesized by pulsed laser ablation in liquid and their use as a selective photoluminescence on–off–on probe for ascorbic acid detection. Carbon 2019, 150, 455–464.
- Park, J.; Bazylewski, P.; Wong, V.; Shah, H.; Fanchini, G. Solvent-free growth of carbon dots by sputter-plasma assisted chemical vapour deposition over large areas. Carbon 2019, 146, 28–35.
- Kalita, H.; Palaparthy, V.S.; Baghini, M.S.; Aslam, M. Electrochemical synthesis of graphene quantum dots from graphene oxide at room temperature and its soil moisture sensing properties. Carbon 2020, 165, 9–17.
- Chen, J.; Long, Z.; Wang, S.; Meng, Y.; Zhang, G.; Nie, S. Biodegradable blends of graphene quantum dots and thermoplastic starch with solid-state photoluminescent and conductive properties. Int. J. Biol. Macromol. 2019, 139, 367–376.
- Alaghmandfard, A.; Sedighi, O.; Rezaei, N.T.; Abedini, A.A.; Khachatourian, A.M.; Toprak, M.S.; Seifalian, A.M. Recent advances in the modification of carbon-based quantum dots for biomedical applications. Mater. Sci. Eng. C 2021, 120, 111756.
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Teng, K.S.; Luk, C.M.; Zeng, S.; Hao, J.; et al. Deep Ultraviolet Photoluminescence of Water-Soluble Self-Passivated Graphene Quantum Dots. ACS Nano 2012, 6, 5102–5110.
- Sangam, S.; Gupta, A.; Shakeel, A.; Bhattacharya, R.; Sharma, A.K.; Suhag, D.; Chakrabarti, S.; Garg, S.K.; Chattopadhyay, S.; Basu, B.; et al. Sustainable synthesis of single crystalline sulphur-doped graphene quantum dots for bioimaging and beyond. Green Chem. 2018, 20, 4245–4259.
- Mahesh, S.; Lekshmi, C.L.; Renuka, K.D.; Joseph, K. Simple and Cost-Effective Synthesis of Fluorescent Graphene Quantum Dots from Honey: Application as Stable Security Ink and White-Light Emission. Part. Part. Syst. Charact. 2015, 33, 70–74.
- Kalita, H.; Mohapatra, J.; Pradhan, L.; Mitra, A.; Bahadur, D.; Aslam, M. Efficient synthesis of rice based graphene quantum dots and their fluorescent properties. RSC Adv. 2016, 6, 23518–23524.
