Shiga toxins (Stxs) are classic bacterial toxins and major virulence factors of toxigenic Shigella dysenteriae and enterohemorrhagic Escherichia coli (EHEC). These toxins recognize a glycosphingolipid globotriaosylceramide (Gb3/CD77) as their receptor and inhibit protein synthesis in cells by cleaving 28S ribosomal RNA. They are the major cause of life-threatening complications such as hemolytic uremic syndrome (HUS), associated with severe cases of EHEC infection, which is the leading cause of acute kidney injury in children.
- Shiga toxin
- EHEC
- Shigella
- hemolytic uremic syndrome
- Gb3
- LAPTM4A
- TM9SF2
- immunotoxin
- bacterial toxins
- toxins
1. Introduction
Shiga toxin (Stx) was named after Japanese microbiologist Kiyoshi Shiga, who identified and characterized Shigella dysenteriae in 1897 [1]. Among Shigella dysenteriae strains, serotype 1 is the toxigenic one that expresses Stx [2]. In 1977, a cytotoxic toxin from Escherichia coli (E. coli) isolates was discovered by Konowalchuck et al., initially named verotoxin or E. coli cytotoxin for its ability to kill cultured Vero cells [3]. By the early 1980s, O’Brien et al. recognized that E. coli isolates express toxins highly similar to Stx; they called them Shiga-like toxins and designated the E. coli isolates Shiga-like toxin-producing E. coli (STEC) [4]. Among STEC, the strains associated with human diseases are also known as enterohemorrhagic E. coli (EHEC). It was soon realized that these toxins belong to the same Stx family and can be divided into two serotypes: Stx1 is almost identical to the prototype Stx in Shigella dysenteriae, while Stx2 shares ~56% protein sequence identity with Stx [5].
Each year, in the United States, there are approximately 265,000 cases of EHEC infection [6], which usually starts with diarrhea and can develop into dysentery and hemorrhagic colitis. In severe cases, life-threatening complications such as hemolytic uremic syndrome (HUS) and neurological disorders can occur. HUS is most commonly associated with EHEC serotype O157:H7 infections, and Stx is the major cause [7]. HUS has the highest incidence in children and the elderly [8][9][8,9] and is the major reason for acute kidney injury in children [10]. Patients have typically developed irreversible vascular damage by the time symptoms appear, and there is no specific treatment [7].
Investigations over the past 40 years have elucidated the molecular mechanism underlying the toxicity of Stx, established its central role in pathogenesis, and shed light on many key cellular functions. Furthermore, Stx and Stx fragments have also been utilized as tools for biomedical applications. Many topics in Stx biology and applications have been covered by excellent in-depth reviews in recent years [9][11][12][13][14][15][16][17][18][19][20][21][22][9,11,12,13,14,15,16,17,18,19,20,21,22].
2. Stx Structure and Function
Stx is an AB5 toxin, comprising an enzymatic A subunit (Stx-A, 32 kDa) and five identical B subunits (Stx-B, 7.7 kDa) that form a pentamer. The A subunit connects to the pentamer noncovalently by inserting its C-terminal region into the central hole [23][24][23,24] (Figure 1). The A subunit is an RNA N-glycosidase, which inhibits protein synthesis by cleaving 28S ribosomal RNA [25][26][27][25,26,27]. The B subunit is responsible for binding to receptors. The glycosphingolipid globotriaosylceramide (Gb3, also known as CD77) is the major receptor of Stx [28]. Crystal structural studies using Stx1-B and a trisaccharide analog of Gb3 revealed three Gb3 binding sites per B subunit (Figure 1). Thus, one Stx can bind up to 15 Gb3 simultaneously [29]. After clustering Gb3 on cell surfaces, Stx is internalized by clathrin-dependent and -independent endocytosis pathways [30][31][30,31], retrogradely sorted into the trans-Golgi network (TGN) and further into the endoplasmic reticulum (ER), bypassing the late endocytic pathway [32][33][34][35][36][32,33,34,35,36]. The A subunit is further processed by host furin and furin-like proteases (cleaving between R251 and M252 of Stx1 and between R250 and A251 of Stx2) into the enzymatic piece A1 (27.5 kDa) and the linker piece A2 (4.5 kDa), connecting to the B subunit. The A1 and A2 domains remain connected by a single disulfide bond, which is reduced within the ER. The A1 domain is then released from the ER into the cytosol through the ER-associated protein degradation (ERAD) pathway to inhibit protein synthesis [14][37][38][14,37,38].
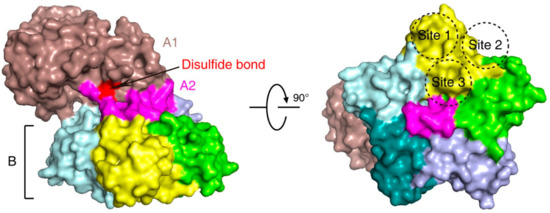
Figure 1. The structure of Shiga toxin: an A subunit, which is cleaved into an enzymatic piece A1 (colored brown) and a linker piece A2 (colored magenta), and five B subunits (PDB: 4M1U). The disulfide bond connecting A1 and A2 pieces is colored red. In the bottom view (right panel), three receptor binding sites on one B subunit (colored yellow) are shown.
3. Stx Subtypes
Stx1 and Stx2 each have multiple subtypes classified based on protein sequence variations. Three subtypes of Stx1 (Stx1a, Stx1c, and Stx1d) and eleven subtypes of Stx2 (Stx2a–Stx2k) have been reported so far [39][40][41][42][39,40,41,42]. Some STEC strains express only one type of Stx, while others may express multiple types simultaneously [39]. Protein stability, toxin potency, receptor preference, symptom severity, and related host reservoirs may vary among subtypes [39][43][39,43]. For example, strains that produce Stx2a, Stx2c, and Stx2d are often associated with colitis and HUS in human infections. STEC producing Stx2b, Stx2e, Stx2f, and Stx2g are usually associated with animal infections, such as in deer, pigs, pigeons, and cattle [17][41][44][45][17,41,44,45]. Stx2e is the common subtype causing the edema disease of swine [46]. Strains producing Stx2f are frequently found in pigeons and other birds [47][48][49][47,48,49]. In 2018, Stx2h and Stx2i were identified in STEC strains isolated from wild marmots and shrimp, respectively [40][50][40,50]. Two additional new subtypes, Stx2j and Stx2k, have recently been reported but have yet to be broadly accepted. Stx2k was isolated from bacterial strains of various sources, including animals (goats and pigs), raw meat (beef and mutton), and human patients with diarrhea [41][42][41,42].
Stx1 and most Stx2 subtypes recognize the Gb3 receptor. However, Stx2e binds preferentially to globotetraosylceramide (Gb4), which is synthesized by adding N-acetylgalactosamine to Gb3 [51]. Toxicity differences among Stx subtypes are partially ascribed to differences in receptor binding, and it is believed that the low dissociation rate of Stx2a with receptors may lead to greater toxicity due to longer toxin uptake [52]. This may be one reason why Stx2a, among all Stx2 subtypes, is implicated in most HUS cases [53].
