The pathogenesis of arterial hypertension is multifactorial, one of the components being represented by incorrect eating habits. In particular, excessive salt and sugar intake can contribute to the onset of hypertension in children, particularly in subjects with excess weight. The recent modification of dietary styles and the current very wide availability of salt and sugar has led to an exponential increase in the consumption of these two nutrients. The dietary intake of salt and sugar in children is in fact much higher than that recommended by health agencies. The purpose of the review is to explore the relationship between an excessive dietary intake of salt and sugar and the onset of arterial hypertension in children and to show the most important clinical studies that demonstrate the association between these two nutrients and arterial hypertension in pediatric age.
The pathogenesis of arterial hypertension is multifactorial, one of the components being represented by incorrect eating habits. In particular, excessive salt and sugar intake can contribute to the onset of hypertension in children, particularly in subjects with excess weight. The recent modification of dietary styles and the current very wide availability of salt and sugar has led to an exponential increase in the consumption of these two nutrients. The dietary intake of salt and sugar in children is in fact much higher than that recommended by health agencies. The purpose of the entry is to explore the relationship between an excessive dietary intake of salt and sugar and the onset of arterial hypertension in children and to show the most important clinical studies that demonstrate the association between these two nutrients and arterial hypertension in pediatric age.
- children
- hypertension
- obesity
- salt
- sodium
- fructose
- uric acid
1. Introduction
The recent modification of the styles of diet and the current very wide availability of salt and sugar have led to an exponential increase in the consumption of these two nutrients.The term "salt" unequivocally refers to sodium chloride, the main source of sodium intake, in addition to the less significant amount of sodium naturally contained in food. Data from the NHANES 2003–2010 showed that more than 80% of children aged 1-5 years exceeded their sodium Upper Intake Levels
[1]
. An Italian study provided evidence that in a large sample of children aged 6-36 months the intake of sodium and free carbohydrates was high. In particular, most of the children aged ≥12 months were taking more sodium than the daily upper limit of 1000 mg suggested by the Italian guidelines
[2]
. In older Italian children, the estimated intake of salt was about 7.0 g per day, which means that they had a consumption higher than the recommended standard dietary target
[3]
. The largest share of sugar is introduced in the diet as sucrose (the common table sugar) and high fructose sweetening syrups (HFCS) used widely, but not exclusively, in soft drinks.In children, the sugars in honey and fruit juices may also have a certain quantitative importance, while the use of fructose alone, erroneously perceived as a natural sweetener, is becoming increasingly popular. Studies on fructose intake in the general population are few. The most significant data are provided by the NHANES, relating to the population of the United States in the years 1999-2004, which shows that the average daily consumption of fructose per capita was 49 grams (of which only 8 grams provided by fruits). In the younger population, however, the intake was much higher (75 grams among males aged 15 to 22)
[4]
. Moreover, in the Zuccotti study, very few babies had an intake of free sugar within the guidelines’ recommended value. value
[2]
.
2. Salt and Pathogenesis of Arterial Hypertension
Arterial hypertension is a complex disease at the origin of which genetic, epigenetic, environmental and behavioral factors interact, with different roles in different age stages (Figure1)
[5]
.Several studies show a correlation between dietary salt intake, blood pressure values and morbidity and mortality from cardiovascular disease. It has also been shown that regardless of body weight, gender and age, salt intake is considered a well-established risk factor for hypertension
[6]
. More recently, it has been hypothesized that salt may exert its effect on blood pressure with different and more complex mechanisms. Excessive salt intake would cause alterations in the physiological systems that regulate cardiac, vascular and/or renal function. In the presence of hypertension, in particular in obese subjects, there is an increase in sympathetic nervous system (SNS) activity
[7]
. This autonomic alteration is already present in pediatric age; in fact, greater sympathetic and lower parasympathetic modulation have been described both in hypertensive children
[8]
and adolescents
[9]
. Activation of the SNS leads on the one hand to an increase in sodium reabsorption at the level of the proximal convoluted tubule and on the other hand stimulates the renin angiotensin aldosterone system (RAAS) which, in turn, increases both distal renal sodium reabsorption and sympathetic activity via angiotensin II. In addition, it seems that various genetic, hormonal and neuroendocrine factors are involved in the development of sodium-sensitive arterial hypertension
[10]
. The SNS, RAAS, natriuretic peptides, insulin, leptin and other endothelial mediators with endocrine activity could all influence the sensitivity of blood pressure to salt intake
[11]
.
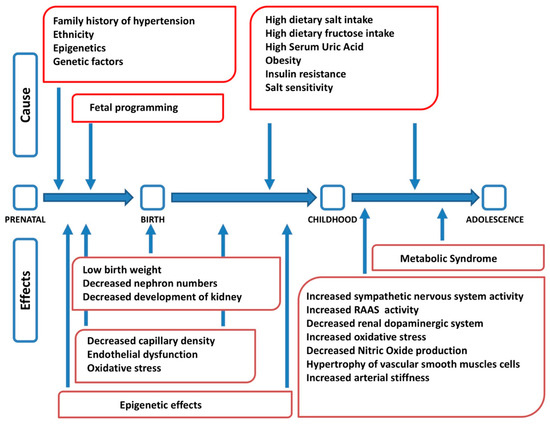
Factors interacting in the pathogenesis of arterial hypertension at different stages of life (modified from
). RAAS: renin angiotensin aldosterone system.
Suckling et al. have suggested that the consumption of foods that are high in sodium leads to a transient increase in plasma sodium concentration which could have toxic effects on the vascular system
[12]
. Plasma sodium concentration may exert an effect on blood pressure by modifying the "stiffness" of endothelial cells. The greater "stiffness" of endothelial cells would lead to a reduction in the activity of nitric oxide synthase (eNOS) and an increase in vascular resistance, with a consequent increase in blood pressure
[13]
. This condition could in turn induce microvascular remodeling and a systemic proinflammatory state leading to microvascular endothelial inflammation, anatomical remodeling and functional abnormalities, as shown in animal models
[14]
. The presence of a pro-inflammatory state involving the endothelium due to an excessive intake of salt has been demonstrated in several studies performed on rodents
and on humans
. These studies show that the negative effects of a high concentration of sodium on the vascular system are mediated by reactive oxygen species (predominantly superoxide, O2−). A high salt content in the diet results in an increase in O2 - which decreases the bioavailability of nitric oxide (NO), which is eliminated by transformation into its radicals. Therefore, a high sodium intake alters endothelial function through a reduced bioavailability of NO. In addition to increasing oxidative stress, a high sodium content can also decrease the antioxidant defense mechanisms by reducing the expression of superoxide dismutase
.
3. Sugar and Pathogenesis of Arterial Hypertension
To evaluate the consequences of sugar consumption on the development of arterial hypertension, both the effect of the total consumption of free sugars and the specific metabolic role of fructose compared to that of glucose should be considered. Furthermore, the relationship between blood pressure and uric acid, the final product of the catabolism of purines (adenosine and cytosine), a metabolic pathway differentiated from the metabolism of sugars, have also to be considered. For clarity, all these aspects will be dealt with separately.
3.1. Free Sugars
In our diet, free sugars, mainly consumed through the intake of sugary drinks (SSB), cause a high calorie intake, both in adults and children, thus favoring the development of excess weight
[23]
. This has been demonstrated both in adolescents
[24]
, and children
[25]
In particular, obesity appears to be the major risk factor for primary arterial hypertension in children
.
3.2. Fructose
After intestinal absorption, fructose can enter to the liver cells without any control and this is the main cause of a series of metabolic alterations
[28]
. The fructose in the hepatocyte is rapidly phosphorylated by phosphofructokinase and transformed into fructose 1 phosphate, with consumption of ATP (Figure 2). In the presence of a high amount of fructose, this entails on the one hand the lowering of the cell's energy level and on the other, due to the degradation of ATP, the production of adenosine and subsequently uric acid. Furthermore, fructose, in addition to inducing insulin resistance, also determines resistance to leptin
[29]
Both conditions inhibit the center of satiety, stimulate food intake , favoring the onset of obesity and, consequently, arterial hypertension
[30]
.It should be emphasized that insulin resistance is a factor independently associated with an increase in blood pressure values, even in children
. There is also an interaction between salt and fructose, because the latter may favor the reabsorption of sodium both in the kidney
[33]
and in the intestine
[34]
, especially when associated with a high salt diet
[35]
. Finally, it has been suggested that continued consumption of fructose would lead to kidney damage
[36]
which, over time, could favor an increase in blood pressure.
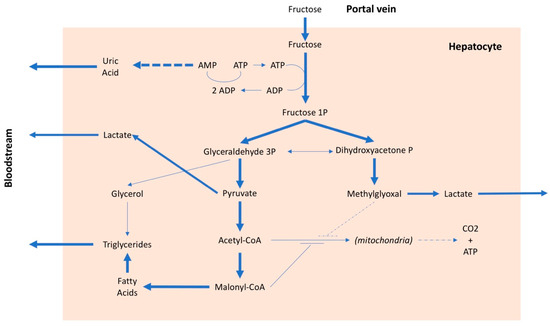
Metabolic pathways of fructose. AMP: adenosine monophosphate, ADP: adenosine diphosphate, ATP: adenosine triphosphate, CO2: carbon dioxide, CoA: coenzyme A, P: phosphate.
3.3. Uric Acid
A large number of studies, both experimental and clinical, have shown an association between uric acid values and arterial hypertension in children. The main cause of increased uricemia in children is excessive consumption of fructose, in particular through SSBs. Uric acid increases oxidative stress
the production of tumor necrosis factor, interleukin 6 and other chemokines, stimulating inflammatory processes especially at the vascular level
[39]
. In addition, uric acid increases insulin resistance
[40]
, favoring the onset of those alterations that characterize the metabolic syndrome, including arterial hypertension. The most important effects of uric acid in the development of hypertension are due to its direct action on arterioles and kidney. At the arteriolar level, uric acid inhibits the production of nitric oxide and the activity of endothelin, favoring the onset of endothelial dysfunction
[41]
.
3. Epidemiological Studies on Salt and Sugar Intake and Arterial Hypertension in Children
Several studies showed a correlation between high salt intake and increased blood pressure values and hypertension prevalence in the pediatric population. A recent meta-analysis showed that sodium intake is associated with blood pressure values in children and adolescents
[42]
. Moreover, a reduction in salt intake causes a decrease in blood pressure and these data provide strong support for the importance of a reduction in salt dietary intake in pediatric age
[43]
. Since blood pressure tracks from childhood to adulthood, these findings suggest that the reduction of sodium intake during childhood and adolescence could lower blood pressure and prevent the development of hypertension later on in life.
Correra-Costa et al. confirmed that the increase in salt consumption leads to an increase in daytime SBP and DBP assessed by 24-hour pressure monitoring [44]. The amount of salt intake in children and adolescents depends on the family's diet. It has been shown that sodium excretion of children is correlated to that of their parents [45][46].
Few studies are available on the effects of dietary sodium intake on blood pressure in the first months of life. He's meta-analysis, which also included a subgroup of three trials performed in infants, showed that a reduction in salt intake led to a significant reduction in SBP
[43]
.Not only salt intake, but also high sugar intake is related to an increase of blood pressure values in children. Evidence has been reported in the literature about a correlation of blood pressure with both fructose consumption (especially taken with SSBs), and serum uric acid values.
In a recent meta-analysis, which included 14 studies performed in children and adolescents, high consumption of SSBs was associated with a significant increase in SBP. Important consumers of SSBs were also more likely to develop hypertension than more modest users
[48]
.Studies that associate high serum uric acid values with the presence of hypertension in children are numerous. Furthermore, an association between hyperuricemia and incident hypertension has been demonstrated. It has been suggested that increases in uricemia in childhood may predict the onset of hypertension in adulthood
[49]
. In a meta-analysis that included 18 prospective studies, the presence of hyperuricemia was associated with a 40% increased risk of incident hypertension
[50]
. Nguyen et al. have highlighted the link between SSBs, uric acid and blood pressure in a population of adolescents
[51]
.
It is generally thought that salt and sugar are taken by children through different foods and separately. Instead, the preparation of many desserts also includes the addition of salt to improve their palatability. Furthermore, current eating habits (western diet) are characterized by a large consumption of both salt and sugar. These findings suggest that if salt intake in children is reduced there may be a reduction also in SSB consumption and this could have a beneficial effect on both body weight and arterial pressure, regardless of the effect directly exerted by the low-sodium diet on blood pressure values
[52]
.
4. Conclusions
Cardiovascular diseases occur in adulthood, but the underlying vascular alterations begin very early in childhood and are related to the presence of risk factors such as arterial hypertension and obesity. Salt and sugar, if taken in excess, are important risk factors for hypertension and obesity. In consideration the importance of early prevention, both at the individual level and at the level of public health, it would be mandatory to apply strategies for limiting the consumption of salt and free sugars and in particular fructose in children.
References
- Tian, N.; Zhang, Z.; Loustalot, F.; Yang, Q.; Cogswell, M.E.; Sodium and potassium intakes among US infants and preschool children, 2003–2010. The American Journal of Clinical Nutrition 2013, 98, 1113-1122, 10.3945/ajcn.113.060012.
- Zuccotti, G.V.; Cassatella, C.; Morelli, A.; Cucugliato, M.C.; Catinello, G.; Del Balzo, V.; Guidarelli, L.; Agostoni, C.; Mameli, C.; Troiano, E.; et al.et al. Nutrient Intake in Italian Infants and Toddlers from North and South Italy: The Nutrintake 636 Study. Nutrients 2014, 6, 3169-3186, 10.3390/nu6083169.
- Campanozzi, A.; Avallone, S.; Barbato, A.; Iacone, R.; Russo, O.; De Filippo, G.; D’Angelo, G.; Pensabene, L.; Malamisura, B.; Cecere, G.; et al.et al. High Sodium and Low Potassium Intake among Italian Children: Relationship with Age, Body Mass and Blood Pressure. PLOS ONE 2015, 10, e0121183, 10.1371/journal.pone.0121183.
- Marriott, B.P.; Cole, N.; Lee, E.; National Estimates of Dietary Fructose Intake Increased from 1977 to 2004 in the United States. The Journal of Nutrition 2009, 139, 1228S-1235S, 10.3945/jn.108.098277.
- Tiu, A.C.; Bishop, M.D.; Asico, L.D.; Jose, P.A.; Villar, V.A.M.; Primary Pediatric Hypertension: Current Understanding and Emerging Concepts. Current Hypertension Reports 2017, 19, 70, 10.1007/s11906-017-0768-4.
- Ekmekcioglu, C.; Blasche, G.; Dorner, T.E.; Too Much Salt and How We Can Get Rid of It. Forschende Komplementärmedizin / Research in Complementary Medicine 2013, 20, 454-460, 10.1159/000357413.
- Grassi, G.; Sympathetic Neural Activity in Hypertension and Related Diseases. American Journal of Hypertension 2010, 23, 1052-1060, 10.1038/ajh.2010.154.
- Genovesi, S.; Pieruzzi, F.U.E.G.; Giussani, M.; Tono, V.; Stella, A.; Porta, A.; Pagani, M.; Lucini, D.; Analysis of Heart Period and Arterial Pressure Variability in Childhood Hypertension. Hypertension 2008, 51, 1289-1294, 10.1161/hypertensionaha.107.109389.
- Farah, B.Q.; Barros, M.V.; Balagopal, B.; Ritti-Dias, R.M.; Heart Rate Variability and Cardiovascular Risk Factors in Adolescent Boys. The Journal of Pediatrics 2014, 165, 945-950, 10.1016/j.jpeds.2014.06.065.
- Galletti, F.; Strazzullo, P.; The blood pressure–salt sensitivity paradigm: pathophysiologically sound yet of no practical value. Nephrology Dialysis Transplantation 2016, 31, 1386-1391, 10.1093/ndt/gfw295.
- Elijovich, F.; Weinberger, M.H.; Anderson, C.A.; Appel, L.J.; Bursztyn, M.; Cook, N.R.; Dart, R.A.; Newton-Cheh, C.H.; Sacks, F.M.; Laffer, C.L; et al. Salt Sensitivity of Blood Pressure.. Hypertension 2016, 68, , e7–e46.
- Suckling, R.J.; He, F.J.; Markandu, N.D.; MacGregor, G.A; Dietary salt influences postprandial plasma sodium concentration and systolic blood pressure. Kidney International 2012, 81, 407-411, 10.1038/ki.2011.369.
- Oberleithner, H.; Riethmüller, C.; Schillers, H.; MacGregor, G.A.; De Wardener, H.E.; Hausberg, M.; Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proceedings of the National Academy of Sciences 2007, 104, 16281-16286, 10.1073/pnas.0707791104.
- Marketou, M.E.; Maragkoudakis, S.; Anastasiou, I.; Nakou, H.; Plataki, M.; Vardas, P.E.; Parthenakis, F.I; Salt‐induced effects on microvascular function: A critical factor in hypertension mediated organ damage. The Journal of Clinical Hypertension 2019, 21, 749-757, 10.1111/jch.13535.
- Lenda, D.M.; Sauls, B.A.; Boegehold, M.A.; Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. American Journal of Physiology-Heart and Circulatory Physiology 2000, 279, H7-H14, 10.1152/ajpheart.2000.279.1.h7.
- Lenda, D.M.; Boegehold, M.A.; Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. American Journal of Physiology-Heart and Circulatory Physiology 2002, 282, H395-H402, 10.1152/ajpheart.0354.2001.
- Guers, J.J.; Kasecky-Lardner, L.; Farquhar, W.B.; Edwards, D.G.; Lennon, S.L; Voluntary wheel running prevents salt-induced endothelial dysfunction: role of oxidative stress.. Journal of Applied Physiology 2019, 126, 502-510, 10.1152/japplphysiol.00421.2018.
- Jody L. Greaney; Jennifer J. Dupont; Shannon L. Lennon-Edwards; Paul W. Sanders; David G. Edwards; William B. Farquhar; Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. The Journal of Physiology 2012, 590, 5519-5528, 10.1113/jphysiol.2012.236992.
- Cavka, A.; Jukic, I.; Ali, M.; Goslawski, M.; Bian, J.-T.; Wang, E.; Drenjancevic, I.; Phillips, S.A.; Short-term high salt intake reduces brachial artery and microvascular function in the absence of changes in blood pressure. Journal of Hypertension 2016, 34, 676-684, 10.1097/hjh.0000000000000852.
- Sessa, W.C.; eNOS at a glance. Journal of Cell Science 2004, 117, 2427-2429, 10.1242/jcs.01165.
- Wray, D.W.; Witman, M.A.H.; Ives, S.J.; Mcdaniel, J.; Trinity, J.D.; Conklin, J.D.; Supiano, M.A.; Richardson, R.S.; Does Brachial Artery Flow–Mediated Vasodilation Provide a Bioassay for NO?. Hypertension 2013, 62, 345-351, 10.1161/hypertensionaha.113.01578.
- Loscalzo, J.; The Identification of Nitric Oxide as Endothelium-Derived Relaxing Factor. Circulation Research 2013, 113, 100-103, 10.1161/CIRCRESAHA.113.301577.
- Malik, V.S.; Schulze, M.B.; Hu, F.B.; Intake of sugar-sweetened beverages and weight gain: a systematic review1–3. The American Journal of Clinical Nutrition 2006, 84, 274-288, 10.1093/ajcn/84.1.274.
- Ebbeling, C.B.; Feldman, H.A.; Chomitz, V.R.; Antonelli, T.A.; Gortmaker, S.L.; Osganian, S.K.; Ludwig, D.S.; A Randomized Trial of Sugar-Sweetened Beverages and Adolescent Body Weight. New England Journal of Medicine 2012, 367, 1407-1416, 10.1056/nejmoa1203388.
- De Ruyter, J.C.; Olthof, M.R.; Seidell, J.C.; Katan, M.B.; A Trial of Sugar-free or Sugar-Sweetened Beverages and Body Weight in Children. New England Journal of Medicine 2012, 367, 1397-1406, 10.1056/nejmoa1203034.
- Genovesi, S.; Giussani, M.; Pieruzzi, F.; Vigorita, F.; Arcovio, C.; Cavuto, S.; Stella, A; Results of blood pressure screening in a population of school-aged children in the province of Milan: role of overweight. Journal of Hypertension 2005, 23, 493-497, 10.1097/01.hjh.0000160203.35910.9f.
- Genovesi, S.; Antolini, L.; Giussani, M.; Brambilla, P.; Barbieri, V.; Galbiati, S.; Mastriani, S.; Sala, V.; Valsecchi, M.G.; Stella, A.; et al. Hypertension, Prehypertension, and Transient Elevated Blood Pressure in Children: Association With Weight Excess and Waist Circumference. American Journal of Hypertension 2010, 23, 756-761, 10.1038/ajh.2010.50.
- Tappy, L.; Lê, K.A.; Metabolic Effects of Fructose and the Worldwide Increase in Obesity. Physiological Reviews 2010, 90, 23-46, 10.1152/physrev.00019.2009.
- Johnson, R.J.; Stenvinkel, P.; Andrews, P.; Sánchez-Lozada, L.G.; Nakagawa, T.; Gaucher, E.; Andres-Hernando, A.; Rodriguez- Iturbe, B.; Jimenez, C.R.; Garcia, G.; et al.et al. Fructose metabolism as a common evolutionary pathway of survival associated with climate change, food shortage and droughts. Journal of Internal Medicine 2019, 287, 252-262, 10.1111/joim.12993.
- Shapiro, A.; Mu, W.; Roncal, C.; Cheng, K.-Y.; Johnson, R.J.; Scarpace, P.J.; Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2008, 295, R1370-R1375, 10.1152/ajpregu.00195.2008.
- Madero, M.; Perez-Pozo, S.E.; Jalal, D.; Johnson, R.J.; Sanchez-Lozada, L.-G; Dietary Fructose and Hypertension. Current Hypertension Reports 2010, 13, 29-35, 10.1007/s11906-010-0163-x.
- Genovesi, S.; Brambilla, P.; Giussani, M.; Galbiati, S.; Mastriani, S.; Pieruzzi, F.; Stella, A.; Valsecchi, M.G.; Antolini, L.; Insulin resistance, prehypertension, hypertension and blood pressure values in paediatric age. J Hypertens 2012, 30, 327-35, 10.1097/HJH.0b013e32834e4aaa..
- Jayalath, V.H.; De Souza, R.J.; Ha, V.; Mirrahimi, A.; Blanco-Mejia, S.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; Wolever, T.M.S.; Beyene, J.; et al.et al Sugar-sweetened beverage consumption and incident hypertension: a systematic review and meta-analysis of prospective cohorts. The American Journal of Clinical Nutrition 2015, 102, 914-921, 10.3945/ajcn.115.107243.
- Gordish, K.L.; Kassem, K.M.; Ortiz, P.A.; Beierwaltes, W.H.; Moderate (20%) fructose-enriched diet stimulates salt-sensitive hypertension with increased salt retention and decreased renal nitric oxide. Physiological Reports 2017, 5, e13162, 10.14814/phy2.13162.
- Cirillo, P.; Gersch, M.S.; Mu, W.; Scherer, P.M.; Kim, K.M.; Gesualdo, L.; Henderson, G.N.; Johnson, R.J.; Sautin, Y.Y.; Ketohexokinase-Dependent Metabolism of Fructose Induces Proinflammatory Mediators in Proximal Tubular Cells. Journal of the American Society of Nephrology 2009, 20, 545-553, 10.1681/asn.2008060576.
- Fang, J.; Alderman, M.H.; Serum Uric Acid and Cardiovascular Mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey.. JAMA 2000, 283, 2404-2410.
- Sautin, Y.Y.; Nakagawa, T.; Zharikov, S.; Johnson, R.J.; Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. American Journal of Physiology-Cell Physiology 2007, 293, C584-C596, 10.1152/ajpcell.00600.2006.
- Chen, L.; Lan, Z.; Lin, Q.; Mi, X.; He, Y.; Wei, L.; Lin, Y.; Zhang, Y.; Deng, X.; Polydatin ameliorates renal injury by attenuating oxidative stress-related inflammatory responses in fructose-induced urate nephropathic mice. Food and Chemical Toxicology 2013, 52, 28-35, 10.1016/j.fct.2012.10.037.
- Kanbay, M.; Segal, M.; Afsar, B.; Kang, D.-H.; Rodriguez-Iturbe, B.; Johnson, R.J.; The role of uric acid in the pathogenesis of human cardiovascular disease. Heart 2013, 99, 759-766, 10.1136/heartjnl-2012-302535.
- Choi, Y.; Yoon, Y.; Lee, K.; Hien, T.T.; Kang, K.W.; Kim, K.; Lee, J.; Lee, M.; Lee, S.M.; Kang, D.; et al.et al. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. The FASEB Journal 2014, 28, 3197-3204, 10.1096/fj.13-247148.
- Su, H.-Y.; Yang, C.; Liang, D.; Liu, H.-F.; Research Advances in the Mechanisms of Hyperuricemia-Induced Renal Injury. BioMed Research International 2020, 2020, 1-12, 10.1155/2020/5817348.
- Magali Leyvraz; Angeline Chatelan; Bruno R Da Costa; Patrick Taffé; Gilles Paradis; Pascal Bovet; Murielle Bochud; Arnaud Chiolero; Sodium intake and blood pressure in children and adolescents: a systematic review and meta-analysis of experimental and observational studies. International Journal Of Epidemiology 2018, 47, 1796-1810, 10.1093/ije/dyy121.
- Feng J. He; Graham A. MacGregor; Importance of Salt in Determining Blood Pressure in Children. Hypertension 2006, 48, 861-869, 10.1161/01.hyp.0000245672.27270.4a.
- Correia-Costa, L.; Cosme, D.; Nogueira-Silva, L.; Morato, M.; Sousa, T.; Moura, C.; Mota, C.; Guerra, A.; Albino-Teixeira, A.; Areias, J.C.; et al.et al. Gender and obesity modify the impact of salt intake on blood pressure in children. Pediatric Nephrology 2015, 31, 279-288, 10.1007/s00467-015-3210-7.
- Cotter, J.; Cotter, M.J.; Oliveira, P.; Cunha, P.; Torres, E.; Polonia, J.; Comparison of Salt Intake in Children to that of their Parents. Nephron 2019, 142, 284-290, 10.1159/000499344.
- Cuadrado-Soto, E.; Peral-Suarez, Á.; Rodríguez-Rodríguez, E.; Aparicio, A.; Andrés, P.; Ortega, R.M.; López-Sobaler, A.M.; The association of parents’ behaviors related to salt with 24 h urinary sodium excretion of their children: A Spanish cross-sectional study. PLOS ONE 2019, 14, e0227035, 10.1371/journal.pone.0227035.
- Viazzi, F.; Antolini, L.; Giussani, M.; Brambilla, P.; Galbiati, S.; Mastriani, S.; Stella, A.; Pontremoli, R.; Valsecchi, M.G.; Genovesi, S.; et al. Serum Uric Acid and Blood Pressure in Children at Cardiovascular Risk. Pediatrics 2013, 132, e93-e99, 10.1542/peds.2013-0047.
- Feig,D.I.; Johnson,R.J.; Hyperuricemia in Childhood Primary Hypertension. Hypertension 2003, 42, 247-252, 10.1161/01.hyp.0000085858.66548.59.
- Alper,A.B.; Chen,W.; Yau,L.; Srinivasan,S.R.; Berenson,G.S.; Hamm,L.L; Childhood Uric Acid Predicts Adult Blood Pressure. Hypertension 2005, 45, 34-38, 10.1161/01.hyp.0000150783.79172.bb.
- Grayson,P.C.; Kim,S.Y.; LaValley,M.; Choi,H.K; Hyperuricemia and incident hypertension: A systematic review and metaanalysis. . Arthritis Rheum 2010, 63, 102–110.
- Nguyen,S.; Choi,H.K.; Lustig,R.H.; Hsu,C.-Y.; Sugar-Sweetened Beverages, Serum Uric Acid, and Blood Pressure in Adolescents. The Journal of Pediatrics 2009, 154, 807-813, 10.1016/j.jpeds.2009.01.015.
- Feng J. He; Naomi M. Marrero; Graham A. MacGregor; Salt Intake Is Related to Soft Drink Consumption in Children and Adolescents. Hypertension 2008, 51, 629-634, 10.1161/hypertensionaha.107.100990.
- Nguyen,S.; Choi,H.K.; Lustig,R.H.; Hsu,C.-Y.; Sugar-Sweetened Beverages, Serum Uric Acid, and Blood Pressure in Adolescents. The Journal of Pediatrics 2009, 154, 807-813, 10.1016/j.jpeds.2009.01.015.
- He, F.J.; Marrero, N.M.; MacGregor, G.A.; Salt Intake Is Related to Soft Drink Consumption in Children and Adolescents. Hypertension 2008, 51, 629-634, 10.1161/hypertensionaha.107.100990.
