The disease-preventive and medicinal properties of plant polyphenolic compounds have long been known. As active ingredients, they are used to prevent and treat many noncommunicable diseases. In recent decades, marine macroalgae have attracted the attention of biotechnologists and pharmacologists as a promising and almost inexhaustible source of polyphenols. This heterogeneous group of compounds contains many biopolymers with unique structure and biological properties that exhibit high anti-infective activity. In the present review, the authors focus on the antiviral potential of polyphenolic compounds (phlorotannins) from marine algae and consider the mechanisms of their action as well as other biological properties of these compounds that have effects on the progress and outcome of viral infections.
- Polyphenolic Compounds of Seaweed
1. Introduction
The high virulence of new and recurring viruses and the lack of effective treatments for the diseases caused by them pose a serious challenge to public health systems. The development of highly effective broad-spectrum antiviral drugs with low toxicity and low cost has been one of the major issues in virology and pharmaceutics for many years. In the period of the ongoing COVID-19 pandemic, it has acquired particular relevance and importance and is aimed at creating agents that inhibit the entry and replication of the virus while modulating the body’s defence systems.
The virus reproduction process includes three phases [1]. The first one is adsorption and entry of the virus into the cell, the release of its internal structural components, and modification into a state in which it can cause an infectious process. The attachment of the virus to macroorganism host cells is a specific interaction between the surface proteins of the virus and the receptors located on the surface of host cells. The second phase of reproduction is regulated by complex processes with the expression of the viral genome. Finally, the third stage of reproduction is the release of viral offspring out of the host cell by budding or lysis.
Currently, medicine has a large range of antiviral agents that can have an effect on each of these stages [2]. At the same time, a rapid increase in their number is observed annually due to compounds isolated from terrestrial plants. The possibility of using synthetic and herbal preparations for the treatment of viral diseases is determined by a number of properties, such as a therapeutic effect, the absence or minimum of side reactions, and low toxicity.
Synthetic antiviral drugs act faster and provide, as a rule, the maximum therapeutic effect. However, their disadvantage is a large number of contraindications and side reactions, as well as addiction and the absence of the desired effect in the future. Herbal antiviral drugs have a wide spectrum of action (apart from the antiviral effect, they have anti-inflammatory, antioxidant and immunomodulatory effects), are less toxic or non-toxic in working doses, and have minimal side effects. It is possible that herbal medicine may have potential as a prophylactic agent and even a therapeutic agent for patients with viral infection.
Despite certain advances in chemotherapy of viral diseases, clinical practice faces serious problems such as the emergence of drug-resistant variants of viruses and side effects of antiviral medicines. This circumstance dictates the need to develop new antiviral drugs with different mechanisms of action [3][4].
Studies on compounds with antiviral properties derived from terrestrial and marine plants have shown that, due to their diverse mechanisms of action (antiviral, immunostimulatory, anti-inflammatory and antioxidant), viruses, as a rule, do not acquire resistance to these compounds. Therefore, aquatic organisms producing substances that are sometimes not found in terrestrial plants and have extremely high polyvalent biological activity have attracted the special attention of researchers [5].
The world’s experience in using marine-derived pharmaceuticals shows the enormous potential of marine organisms as raw materials for the creation of original pharmaceutical substances and medicines [6]. Algae, sponges, bacteria, fungi, invertebrates, soft corals, fish, etc. can be sources of new antiviral pharmacological compounds of marine origin [7][8][9]. A number of compounds from these organisms are commercially available on the pharmaceutical market worldwide as an alternative to antiviral drugs [10].
2. General Characteristics of the Polyphenolic Compounds of Seaweed
Marine macroalgae are a unique raw material for obtaining a wide range of natural compounds with interesting and useful biological properties. Their composition is characterised by a rich content of mineral and organic substances. For thousands of years, these hydrobionts have been actively used by humans and animals for food and have served as a valuable source of proteins, fats, carbohydrates, dietary fibre, minerals, etc.
Regular consumption of seaweed can reduce the risk of various pathologies, including cancer, metabolic and degenerative disorders, infectious diseases and cardiovascular diseases. The highest antiviral activity, as shown by numerous experimental studies, is possessed by polyphenolic compounds and sulphated polysaccharides. The content of biologically active substances in seaweed varies depending on the season and region of collection and is largely determined by the type of algae. According to the presence of specific pigments, macroalgae are divided into three main groups: brown (Phaeophyceae), green (Chlorophyta) and red (Rhodophyta) seaweed.
Polyphenols (PPs)—highly hydrophilic secondary metabolites of seaweed—are one of the most numerous groups of substances in the plant kingdom. Macro- and microalgae, as well as cyanobacteria accumulate PPs, in particular, phloroglucinol and its polymers, i.e., phlorotannins [11]. Bromophenols, phenolic acids and flavonoids account for the largest proportion of phenolic compounds found in red and green seaweed [12]. Phlorotannins (PTs) are a heterogeneous group of unique polyphenolic compounds differing in structure and degree of polymerisation and are found only in brown seaweed (up to 25% of dry weight) [13][14]. The largest amount of PTs accumulates in fucus brown seaweed [15][16][17][18]. PTs consist of monomeric units of phloroglucinol (1,3,5-hydroxybenzene), from which more than 700 natural variations of these compounds have been obtained and used in various fields [19] (Figure 1).
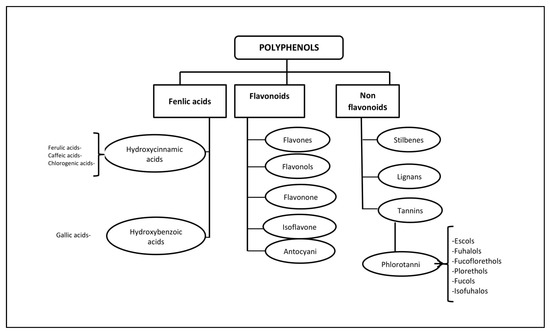
Figure 1. Classification of polyphenols and six main subclasses of seaweed phlorotannins.
Unlike the tannins of terrestrial plants, PTs have a wider range of molecular weights, from 126 Da to 650 kDa (more often from 10 to 100 kDa). The characterisation of PPs is difficult due to heterogeneity both in molecular weight and in the level of isomerisation [20][21]. There is still little information about endogenous digestion and microbial catabolism of these compounds [22]. It is known that about 90–95% of dietary PPs reach the intestine unchanged [23], where, as a result of metabolism and biotransformation, low molecular weight compounds with less chemical heterogeneity are formed than in the original [24].
Some PTs in seaweed can be sulphated or halogenated [25]. The biosynthesis of PTs is carried out through the acetate-malonate pathway in the Golgi apparatus in the perinuclear region of the cell. They are usually not secreted, and cell destruction is necessary to obtain them. In terms of structure and polymeric properties, PTs represent an extensive group of molecules that differ in the nature of the bonds between phloroglucinol and hydroxyl groups (Figure 1). Depending on the type of bond between the monomers, phlorotannins are divided into four subclasses: phlorethols and fuhalols, fucols, fucophlorethols, and eckols and carmalol [26][27]. These compounds exist mainly in a soluble form or in a bound state with components of the cell wall, which ensure its integrity as well as protection from herbivores and oxidative stress.
Terrestrial plants produce tannins that are composed of only three or four phenolic rings, while seaweed PTs are composed of eight phenolic rings. PTs have very strong antioxidant properties as phenolic rings act as electron traps for free radicals [12]. A positive correlation has been noted between the antioxidant activity of PTs and the number of hydroxyl groups present in the structure of the compound [28]. PTs inhibit α-glucosidase, which is responsible for the stepwise removal of terminal glucose residues from the N-glycan chains associated with glycoprotein maturation. Most glycoproteins of the viral environment contain N-linked glycans, and α-glucosidase inhibitors have been proposed as useful broad-spectrum antiviral agents based on their activity against enveloped viruses [29]. The anti-inflammatory [30], antiallergic [31], antiviral [32] and antitumor [33] properties, as well as antidiabetic and radioprotective effects [34] of these biologically active compounds have been demonstrated.
Methods for obtaining PTs, their identification and establishment of the structure are described in sufficient detail in numerous works [13][14][35]. The main difficulty in the extraction of PPs arises from their presence in the form of complex polymer mixtures, for example, with polysaccharides, which, along with proteins, are the main covalently bound component of the algal cell wall [36].
3. Interaction of Seaweed Polyphenols with Enveloped and Nonenveloped Viruses
Resistance of viruses to adverse environmental factors is determined by their structure. There are viruses with simple and complex structure. Simple, or nonenveloped, viruses are composed of a nucleic acid and protein envelope (capsid). Complex, or enveloped, viruses are surrounded by a lipoprotein envelope (supercapsid) over the capsid, which makes them more vulnerable to adverse environmental factors [5][6][23].
Enveloped and nonenveloped viruses also differ in resistance to chemicals, including disinfectants. Thus, the lipoprotein-enveloped influenza, parainfluenza viruses and coronaviruses are low-resistant pathogens; adenoviruses are more resistant; and the nonenveloped rhinovirus is one of the very resistant pathogens such as poliovirus and hepatitis A virus [37].
A.
Interaction of Polyphenols of Seaweed with Enveloped Viruses
In recent years, intensive studies of the antiviral activity of polyphenolic compounds from terrestrial plants, as well as from various marine aquatic organisms, including macroalgae, have been carried out [38][39][40][41]. Mainly enveloped viruses are reported as sensitive to PPs. Figure 2 shows the targets of the enveloped virus that can be affected by plant polyphenols.
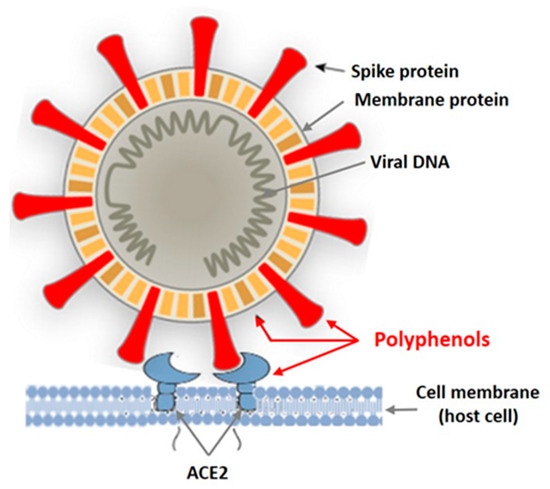
Figure 2. Targets of the enveloped virus for polyphenols of marine and terrestrial plants.
Tannins are known as powerful protein inactivators, including viral ones. M. Wink [38][39] showed that plant tannins form several hydrogen and ionic bonds when interacting with a virus protein, which act on the three-dimensional structure of the protein, suppressing its activity. As land plant tannins and algal tannins are similar in structure, the mechanisms of their interaction with enveloped viruses are probably similar. Polyphenols bind to viral envelope proteins, preventing the pathogen from interacting with the host cell.
Coronaviruses are enveloped viruses. To date, 39 known species of enveloped viruses are known, with each species comprising dozens and hundreds of strains. In addition to the nucleic acid and the associated structurally protective protein (in coronaviruses, it is the N protein), they also have a membrane envelope. The life cycle of coronaviruses provides many potential targets for antiviral intervention. Approaches to the development of anti-coronavirus drugs include exposure to the virus during the steps of penetration and entry of a viral particle into a cell, replication of viral nucleic acid, release of virion from a cell and effects on the cellular targets of the host.
One of the members of coronaviruses is the porcine epidemic diarrhoea virus (PEDL). First recorded in the United States in 2013, it has caused major economic damage in many countries due to the significant mortality of newborn piglets. The PEDL infects the cells lining the pig’s small intestine, causing severe epidemic diarrhoea and dehydration [40][41].
The causative agent was investigated using electron and immunoelectron microscopy. It was shown to differ from the coronaviruses known by that time: the porcine transmissible gastroenteritis (TGS) virus and porcine hemagglutinating encephalomyelitis. Kwon et al. [42] found an antiviral effect of ethanol extract and five phlorotannins obtained from the brown alga Ecklonia cava against the PEDL. The extracted compounds were identified as phloroglucinol (1), eckol (2), 7-phloreckol (3), phlorofucofuroeckol (4) and dieckol (5). Compounds (4) and (5) were present in the ethanol extract from seaweed in sufficiently large amounts [29].
To assess the antiviral activity of the compounds in vitro, two strategies were used: blocking the virus’ binding to cells (obtaining the effect of treatment simultaneously with the infection) and inhibiting the virus’ replication (obtaining the effect of treatment after the infection). The use of the former experimental scheme made it possible to establish that compounds (2–5) have an antiviral activity against the PEDL with the 50% inhibitory concentration (IC50) in the range from 10.8 ± 1.4 to 22.5 ± 2.2 μM. Compounds (2–5) completely blocked the binding of virus protein to sialic acid at concentrations lower than 36.6 μM by inhibiting hemagglutination. The results of the use of the latter experimental design showed that these compounds also blocked the virus’ replication with IC50 values of 12.2 ± 2.8 and 14.6 ± 1.3 μM, respectively, by inhibiting the synthesis of RNA and virus protein, but did not suppress the viral protease [28][30][31]. Regarding the cytotoxicity of the extract, the CC50 was 533.6 μg/mL and ranged from 374.4 to 579 μM for compounds (4) and (5). The experiments were carried out using the lowest toxic (>90% cell viability) concentrations of the extract [30][31].
The PT activity was distributed as follows: dieckol (16.6 ± 3.0 μM) > 7 phlorofucofuroeckol (18.6 ± 2.3 μM) > eckol (22.5 ± 2.3 μM). Phloroglucinol was inactive. PT activity was distributed as follows: dieckol (16.6 ± 3.0 μM) > 7 phlorofucofuroeckol (18.6 ± 2.3 μM) > eckol (22.5 ± 2.3 μM). Phloroglucinol was inactive. PT activity was influenced by the number of hydroxyl groups. Thus, oligomerisation and the existence of the cyclopentane ring may be important for the manifestation of antiviral activity. The authors recommend phlorofucofuroeckol and dieckol from the brown seaweed E. cava as potential agents that act on the most important targets of PEDV.
B. Interaction of PTs of Algae with Nonenveloped Viruses
However, enveloped viruses are not only sensitive to the action of plant phenolic compounds, in particular tannins. Ueda et al. [43] found, for example, that persimmon extracts containing about 22% tannin reduced the infectivity of nonenveloped viruses (poliovirus, Coxsackie virus, adenovirus, rotavirus, feline calcivirus and mouse norovirus) by more than 4 log. The authors believe that the main mechanism of the antiviral action of the extract is associated with the aggregation of viral proteins, as evidenced by the competitive suppression of the antiviral effect by BSA. Algal phlorotannins also have an inhibitory effect on nonenveloped viruses. Such results are noted for human papillomavirus (HPV). As an example, we consider HPV, a small, nonenveloped virus possessing a capsid with cubic symmetry and containing two proteins, L1 and L2. The former is the main capsid protein that makes up more than 80% of the capsid material, forming blocks (capsomeres) from which the capsid is built. Anti-L1 antibodies exhibit virus-neutralising activity. L2 is a minor protein involved in the capsid stabilisation and linking with the genome [44]. The genital infection caused by the human papillomavirus (HPV) is the most common sexually transmitted disease. Most cases of cervical cancer are associated with this infection. Therefore, there is considerable interest in new effective non-reactogenic drugs for the treatment and prevention of this disease.
Kim and Kwak [44] investigated the effect of PT from the brown alga E. bicyclis on HPV. It was found that the seaweed EtOH extract exhibited antiviral activity against HPV 16PVs and HPV 18PVs. Then, the extract was sequentially separated with CH2Cl2, EtOAc and n-BuOH. The most active EtOAc fraction was used for chromatographic separation and resulted in the isolation of eckol, 8,8′-bieckolm 6,6′-bieckol and phlorofucofuroeckol A- Antiviral activity was assessed in 293T cell culture using bioluminescence. All compounds showed a decrease in the viral load of both viruses at a concentration of 50 μg/mL.
Noroviruses, a nonenveloped type of enterovirus, are considered the leading cause of epidemics of diseases accompanied by vomiting, diarrhoea, mild fever, abdominal cramps and nausea [45][46]. Norovirus is characterised by a long isolation period, low infectious dose, high resistance, considerable diversity and frequent genome mutations. The virus is transmitted through contaminated water or food and is spread by the faecal–oral route following contact with infected materials. The virus has a single-stranded positive sense RNA genome [47]. In recent years, attempts have been made to find harmless means of therapy and prevention of infection among terrestrial and marine organisms and algae [48]. To this aim, Eom et al. [40] investigated the possibility of using E. bicyclis seaweed extract and its ingredients as an alternative agent against norovirus. The following fractions were obtained from the EtOAc-soluble extract of E. bicyclis: phlorofucofuroeckol A (PFE) and dieckol (DE).
The MeOH extract and its components did not show significant cytotoxicity. The CC50 was 322.48 to 2146.42 μg/mL. The EtOAc extract showed strong antiviral activity and low cytotoxicity. Earlier [40], the authors described the structure of the extract components DE and PFE and their pronounced antiviral properties. PFE inhibits norovirus infection more intensely than DE. The selective index (SI) values for DE and PFE were approximately 20- and 25-fold higher than that of green tea epigallocatechin gallate. The antiviral activity of DE at IC50 was 0.9 ± 0.06, SI—CC50 IC50—550.6 ± 6.09; PFE, IC50—0.9 ± 0.07, SI—668.87 ± 73.06 [49].
The results obtained by the authors indicate that the use of PTs from E. bicyclis seaweed against norovirus infection is promising. They suggested that PTs prevent viruses from attaching to host cells and proposed to conduct an in-depth study of the mechanisms of anti-rotavirus action of these compounds.
An extract and PTs (eckol and PFE) from the seaweed E. cava were used to enhance protection against the nonenveloped RNA haemorrhagic septicaemia virus (VHSV) causing a highly contagious disease of freshwater and marine fish at different ages [41]. Using cell culture from fathead minnow, it was found that the extract and PTs at low concentrations exhibited strong antiviral activity. When cells were treated with the extract and PT simultaneously with the infection, the values increased (46.4–96.4%) as compared with those in the variants of the experiment before (16.5–48.4%) and after the infection (39.5–56, five%). The IC50 for the extract, eckol and PFE were 4.76 μM, 1.97 μM and 0.99 μM, respectively. The effect increased depending on the time of exposure. In in vivo experiments, a seaweed extract, administered orally at different doses to VHSV-infected flounder, increased the survival rate of fish (by 31.57% at a dose of 500 μg/g/day; by 12.5% at 50 μg/g/day) 12.5%) [40].
Thus, not only enveloped, but also nonenveloped viruses, are sensitive to seaweed PTs. The mechanism of action of these compounds towards the former is better known.
References
- Ryu, W.-S. Virus Life Cycle. Mol. Virol. Hum. Pathog. Viruses 2017, 5, 31–45.
- Abdullah, A.A.; Abdullah, R.; Nazariah, Z.A.; Balakrishnan, K.N.; Abdullah, F.F.J.; Bala, J.A.; Mohd-Lila, M.-A. Cyclophilin A as a target in the treatment of cytomegalovirus infections. Antivir. Chem. Chemother. 2018, 26.
- Strasfeld, L.; Chou, S. Antiviral Drug Resistance: Mechanisms and Clinical Implications. Infect. Dis. Clin. N. Am. 2010, 24, 413–437.
- Irwin, K.K.; Renzette, N.; Kowalik, T.F.; Jensen, J.D. Antiviral drug resistance as an adaptive process. Virus Evol. 2016, 2, vew014.
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465.
- Pedrosa, R.; Gaudêncio, S.P.; Vasconcelos, V. XVI International Symposium on Marine Natural Products|XI European Conference on Marine Natural Products. Mar. Drugs 2020, 18, 40.
- Moghaddam, J.A.; Dávila-Céspedes, A.; Kehraus, S.; Crüsemann, M.; Köse, M.; Müller, C.E.; König, G.M. Cyclopropane-Containing Fatty Acids from the Marine Bacterium Labrenzia sp. 011 with Antimicrobial and GPR84 Activity. Mar. Drugs 2018, 16, 369.
- Santhi, L.S.; Vssl, P.T.; Sy, N.; Radha Krishna, E. Bioactive Compounds from Marine Sponge Associates: Antibiotics from Bacillus sp. Nat. Prod. Chem. Res. 2017, 5, 4.
- Riccio, G.; Lauritano, C. Microalgae with Immunomodulatory Activities. Mar. Drugs 2019, 18, 2.
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91.
- Poole, J.; Diop, A.; Rainville, L.-C.; Barnabé, S. Bioextracting Polyphenols from the Brown Seaweed Ascophyllum nodosum from Québec’s North Shore Coastline. Ind. Biotechnol. 2019, 15, 212–218.
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609.
- Имбс, Т.; Звягинцева, Т. ФЛОРОТАННИНЫ - ПОЛИФЕНОЛЬНЫЕ МЕТАБОЛИТЫ БУРЫХ ВОДОРОСЛЕЙ. Биoлoгия мoря 2018, 44, 217–227.
- Heffernan, N.; Smyth, T.J.; Soler-Villa, A.; Fitzgerald, R.J.; Brunton, N.P. Phenolic content and antioxidant activity of fractions obtained from selected Irish macroalgae species (Laminaria digitate, Fucus serratus, Gracillaria gracilis and Codium fragile). J. Appl. Phycol. 2014, 27, 519–530.
- Shibata, T.; Kawaguchi, S.; Hama, Y.; Inagaki, M.; Yamaguchi, K.; Nakamura, T. Local and chemical distribution of phlorotannins in brown algae. Environ. Biol. Fishes 2004, 16, 291–296.
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597.
- Sathya, M.; Kokilavani, R. Phitochemical screening and in vivo antioxidant activity of Saccharum spontaneous linn. Int. J. Pharm. Sci. Rev. Res. 2013, 18, 75–79.
- Manandhar, S.; Luitel, S.; Dahal, R.K. In Vitro Antimicrobial Activity of Some Medicinal Plants against Human Pathogenic Bacteria. J. Trop. Med. 2019, 2019, 1895340.
- Singh, R.; Akhtar, N.; Haqqi, T.M. Green tea polyphenol epigallocatechi3-gallate: Inflammation and arthritis. Life Sci. 2010, 86, 907–918.
- Melanson, J.E.; MacKinnon, S.L. Characterization of Phlorotannins from Brown Algae by LC-HRMS. Methods Mol. Biol. 2015, 1308, 253–266.
- Montero, L.; Sánchez-Camargo, A.P.; García-Cañas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J. Chromatogr. A 2016, 1428, 115–125.
- Swallah, M.S.; Fu, H.; Sun, H.; Affoh, R.; Yu, H. The Impact of Polyphenol on General Nutrient Metabolism in the Monogastric Gastrointestinal Tract. J. Food Qual. 2020, 2020, 1–12.
- Clifford, M.N. Diet-Derived Phenols in Plasma and Tissues and their Implications for Health. Planta Med. 2004, 70, 1103–1114.
- Lewandowska, U.; Szewczyk, K.; Hrabec, E.; Janecka, A.; Gorlach, S. Overview of Metabolism and Bioavailability Enhancement of Polyphenols. J. Agric. Food Chem. 2013, 61, 12183–12199.
- Ragan, M.A.; Glombitza, K.W. Phlorotannins, brown algal polyphenols. Prog. Phycol. Res. 1986, 4, 129–241.
- Pádua, D.; Rocha, E.; Gargiulo, D.; Ramos, A. Bioactive compounds from brown seaweeds: Phloroglucinol, fucoxanthin and fucoidan as promising therapeutic agents against breast cancer. Phytochem. Lett. 2015, 14, 91–98.
- Mannino, A.M.; Micheli, C. Ecological Function of Phenolic Compounds from Mediterranean Fucoid Algae and Seagrasses: An Overview on the Genus Cystoseira sensu lato and Posidonia oceanica (L.) Delile. J. Mar. Sci. Eng. 2020, 8, 19.
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047.
- Rengasamy, K.R.; Aderogba, M.A.; Amoo, S.O.; Stirk, W.A.; Van Staden, J. Potential antiradical and alpha-glucosidase inhibitors from Ecklonia maxima (Osbeck) Papenfuss. Food Chem. 2013, 141, 1412–1415.
- Kim, A.-R.; Shin, T.-S.; Lee, M.-S.; Park, J.-Y.; Park, K.-E.; Yoon, N.-Y.; Kim, J.-S.; Choi, J.-S.; Jang, B.-C.; Byun, D.-S.; et al. Isolation and Identification of Phlorotannins fromEcklonia stoloniferawith Antioxidant and Anti-inflammatory Properties. J. Agric. Food Chem. 2009, 57, 3483–3489.
- Li, Y.; Lee, S.-H.; Le, Q.-T.; Kim, M.-M.; Kim, S.-K. Anti-allergic Effects of Phlorotannins on Histamine Release via Binding Inhibition between IgE and FcεRI. J. Agric. Food Chem. 2008, 56, 12073–12080.
- Ahn, M.-J.; Yoon, K.-D.; Min, S.-Y.; Lee, J.S.; Kim, J.H.; Kim, T.G.; Kim, S.H.; Kim, N.-G.; Huh, H.; Kim, J. Inhibition of HIV-1 Reverse Transcriptase and Protease by Phlorotannins from the Brown Alga Ecklonia cava. Biol. Pharm. Bull. 2004, 27, 544–547.
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A Source of Bioactive Phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327.
- Lee, S.-H.; Jeon, Y.-J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129–136.
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring Ultrasound, Microwave and Ultrasound–Microwave Assisted Extraction Technologies to Increase the Extraction of Bioactive Compounds and Antioxidants from Brown Macroalgae. Mar. Drugs 2020, 18, 172.
- Koivikko, R.; Eranen, J.K.; Loponen, J.; Jormalainen, Y. Variation of phlorotannins among three populations of Fucus vesic-ulosus as revealed by HPLC and colorimetric quantification. J. Chem. Ecol. 2008, 34, 57–64.
- Firquet, S.; Beaujard, S.; Lobert, P.-E.; Sané, F.; Caloone, D.; Izard, D.; Hober, D. Survival of Enveloped and Non-Enveloped Viruses on Inanimate Surfaces. Microbes Environ. 2015, 30, 140–144.
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286.
- Wink, M. Potential of DNA intercalating alcaloids and other plant secondary metabolites against SARS-CoV-2 causing COVID-19. Diversity 2020, 12, 175.
- Venkatesan, J.; Keekan, K.K.; Anil, S.; Bhatnagar, I.; Kim, S.-K. Phlorotannins. Encycl. Food Chem. 2019, 27, 515–527.
- Yang, H.-K.; Jung, M.-H.; Avunje, S.; Nikapitiya, C.; Kang, S.Y.; Ryu, Y.B.; Lee, W.S.; Jung, S.-J. Efficacy of algal Ecklonia cava extract against viral hemorrhagic septicemia virus (VHSV). Fish Shellfish. Immunol. 2018, 72, 273–281.
- Kwon, H.-J.; Ryu, Y.B.; Kim, Y.-M.; Song, N.; Kim, C.Y.; Rho, M.-C.; Jeong, J.-H.; Cho, K.-O.; Lee, W.S.; Park, S.-J. In vitro antiviral activity of phlorotannins isolated from Ecklonia cava against porcine epidemic diarrhea coronavirus infection and hemagglutination. Bioorgan. Med. Chem. 2013, 21, 4706–4713.
- Ueda, K.; Kawabata, R.; Irie, T.; Nakai, Y.; Tohya, Y.; Sakaguchi, T. Inactivation of Pathogenic Viruses by Plant-Derived Tannins: Strong Effects of Extracts from Persimmon (Diospyros kaki) on a Broad Range of Viruses. PLOS ONE 2013, 8, e55343.
- Cho, H.M.; Doan, T.P.; Ha, T.K.Q.; Kim, H.W.; Lee, B.W.; Pham, H.T.T.; Cho, T.O.; Oh, W.K. Dereplication by High-Performance Liquid Chromatography (HPLC) with Quadrupole-Time-of-Flight Mass Spectroscopy (qTOF-MS) and Antiviral Activities of Phlorotannins from Ecklonia cava. Mar. Drugs 2019, 17, 149.
- Khokhlova, N.I.; Kapustin, D.V.; Krasnova, E.I.; Izvekova, I.Y.I. NOROVIRUS INFECTION (SYSTEMATIC REVIEW). J. Infectol. 2018, 10, 5–14.
- La Rosa, G.; Muscillo, M. Molecular detection of viruses in water and sewage. In Viruses in Food and Water; Elsevier: Amsterdam, The Netherlands, 2013; pp. 97–125.
- Atmar, R.L. Noroviruses: State of the Art. Food Environ. Virol. 2010, 2, 117–126.
- Choi, Y.; Kim, E.; Moon, S.; Choi, J.-D.; Lee, M.-S.; Kim, Y.-M. Phaeophyta Extracts Exhibit Antiviral Activity against Feline Calicivirus. Fish. Aquat. Sci. 2014, 17, 155–158.
- Eom, S.H.; Moon, S.Y.; Lee, D.S.; Kim, H.J.; Park, K.; Lee, E.W.; Kim, T.H.; Chung, Y.H.; Lee, M.S.; Kim, Y.M. In vitro antiviral activity of dieckol and phlorofucofuroecko-A isolated from edible brown alga Eisenia bicyclis against murine norovirus. Algae 2015, 30, 241–246.
