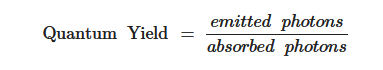Diketopyrrolopyrrole (DPP) organic dyes show an exceptional photophysical features (high-fluorescence quantum yield (FQY), good photochemical and thermal stability) that are essential properties for biological applications. This organic dye pigment of DPP is highly effective as it have shown promising results in various applications of AIE, solid-state emission, bio-imaging, cancer therapy and biorecognition of other essential biological components (biomolecules, proteins, enzymes, mitochondria stains) etc. All these results from the DPPs' high fluorescence quantum yield along with its imperatively low-energy fluorescent probe derivatives. Owing to its outstanding photophysical features, in the last decade numerous research papers have been reported with essentially positive results especially for supramolecular chemistry applications. Therefore in a nutshell, this exciting and attractive research area is presently at its infancy, so great efforts needs to be given to it in order to uncover other potentials which might be hidden that are yet to be known to the scientific communities, especially chemists in order to significantly advance it research horizon higher.
- diketopyrrolopyrrole
- fluorescence
- quantum yield
- aggregation-induced emission
- solid-state emission
- bio-imaging
- cancer therapy
1. Introduction
Fluorescent probes (FPs) are materials possessing fluorescence properties, like fluorescence time, wavelength and emission intensity, changes resulting from specific interactions with target species for sensing and visualizing of some biological molecules as a result of their progressiveness, noninvasiveness and high sensitivity with spatial resolution, hence gaining considerable research interests [1,2,3,4,5][1][2][3][4][5]. Over the years, organic dyes (ODs) have been used in the fluorescence research field to develop probes for different kinds of applications, such as optoelectronics [6], organic electronics light-emitting devices like OLEDs, solar cells and OFETs [7,8[7][8][9][10][11],9,10,11], with detection of essential biological components, bio-imaging and even therapy of cancer/tumor cells [12,13,14,15][12][13][14][15]. Such utilized novel FPs are mostly from coumarin, rhodamine, fluorescein, cyanine, boron-dipyrromethane (BODIPY) and diketopyrrolopyrrole (DPP). This is because these fluorogenic core units possess outstanding photoluminescence (PL) properties [16].
Coumarin dyes absorb and emit in the visible region high-energy wavelength (blue-shift) [17], thus limiting their biological applications. High-fluorescence quantum yields (FQYs) and good photostability are characteristic features of fluorescein [18,19][18][19] and rhodamine [20] dyes, but such dyes have a poor role to play biologically, as their absorption/emission maxima takes place mostly below 600 nm [16], hence limiting their biological applications. Undoubtedly, cyanine dyes biologically have a good role to play [21,22,23,24,25][21][22][23][24][25]; however, their moderately low photostability hindered their biological applications, as well in relation to fluorescein and rhodamine dyes. Derivatives of BODIPY possessed excellent absorption/emission maxima along with high molar absorptivity, thus attracting research interest, too [26,27][26][27]. BODIPY and DPP dyes both possessed features suitably useful in supramolecular chemistry (SC) the (high FQY, excellent absorption/emission and very good photostability).
However, during the past decade, DPP dyes have auspiciously attracted considerable research attention in this research field, especially in biological systems. This is as a result of fluorescent DPPs’ inherent absorption and emission properties to have predominantly moves red-shift in an electromagnetic spectrum. Essentially important is the fact the typical DPP skeleton without any optimization fluoresce strongly above 500 nm (green-yellow) [28], and proper functionalization moves it easily to red-shift to the near-infrared region (NIR) high wavelength (low-energy) with improved absorption/emission above 600 nm, and even far red to (800–900) nm, hence establishing strongly optimized DPP fluorophores with narrow NIR and far-red absorption at lower energy. This is because fluorophores fluorescing at higher wavelengths are essentially important biologically, as their cells and water absorption, autofluorescence and light scattering are greatly reduced, thus facilitating light penetrability deeply and hence boosting their therapeutic/diagnostic functions more effectively. Moreover, DPP dyes are deeply bright red dyeing pigments, and, accordingly, now their molar absorptivity is relatively high, ~76,000 M−1 cm−1, close to that of BODIPY, displaying ~80,000 M−1 cm−1. These mentioned ODs’ photophysical properties are briefly summarized in Table 1, below.
Table 1. Compared photophysical features of diketopyrrolopyrrole (DPP) with mostly used organic dyes.
Organic Dyes | Abs./Em. (nm) | Abs./Em. Max (nm) | Photostability | Molar Absorptivity | (M −1 cm−1) | FQY | Reference | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
DPP | 400–500/500–600 | 500>/650> | Extremely High | 76,000 | 0.4–0.9 | This work | ||||||||||||||||
Rhodamine | 470–528/510–610 | 530/621 | Good | 116,000 | 0.95 |
[16] |
||||||||||||||||
Fluorescein | 400–550/460–700 | 494/512 | High | 80,000 | 0.92 |
[16] |
||||||||||||||||
Cyanine | 400–800/500–800 | 473/767 | High | 100,000 | 0.12–0.67 |
[16] |
||||||||||||||||
Coumarin | 320–520/450–590 | 420/460 | Moderately | 20,000 | 0.78 |
[16] |
||||||||||||||||
BODIPY | 500–645/506–760 | 503/512 | Extremely High | 80,000 | 0.92 |
[16] |
Abs, absorption; Em, emission; DPP, diketopyrropyrrole; BODIPY, boron-dipyrromethane; FQY, high-fluorescence quantum yield.
DPP-based FPs has a very high FQY, good light and are thermally stable (excellently photostable), thus serving as unique building blocks for various applications primarily for field-effect transistors [29,30,31][29][30][31] and photovoltaic cells [32,33,34][32][33][34] due to their substantial semiconducting properties. Different DPP-based FPs are being reported from the literature for numerous applications which can be categorically classified as; those for analytes (ions, ROS, thiols, pH, CO2 and H2) [35,36,37][35][36][37] molecular/and or bio-imaging probes [38], the near-infrared (NIR) dyes [39] and even luminescent polymer probes [40], etc. However, despite such developmental progress for this class of high performing DPP-based FPs, yet their potentials to detect essentially important biological molecules is still infantile. Considering the DPP’s exceptional photophysical features, recently efforts are being made towards advancing its FPs and investigating their biosensing capability [41,42,43][41][42][43] in order to lessen such challenges and difficulties precisely for SC using various strategies owing to the low-energy of its optimized fluorophore essentially useful, especially in solutions and membrane-like systems.
1.1. Overview of the Typical DPP Fluorophore
The DPP skeletal unit possesses actively different functionalities (Figure 1), the bis(lactam) unit having functional groups of alkenes and carbonyl (ketones) coupled with secondary amidic hydrogen (N-H), which undergoes different functionalization to generate various derivatives of the DPP materials. The DPP core unit is obviously known for its high electron affinity, thus making it suitable for both electrophilic and nucleophilic substitution reaction. DPPs have been recently attracting considerable research attention, simply due to their outstanding and distinctive photophysical features, as summarized in Table 1, above. Therefore, they are unique semiconductor building blocks for both hybrid and purely organic materials, with remarkable semiconducting properties primarily for Organic Field-Effect Transistors (OFETs), photovoltaic devices and other relevant organic optoelectronics application. The typical DPP fluorophore absorbs and fluoresces strongly, exceeding 500 nm (green-yellow emission) without modification, and proper optimization moves it easily red-shift; thus, such modified low-energy fluorophores are useful for biosensing and bio-imaging applications, as briefly introduced and discussed. These features have caused DPP to attract considerable research attention for the past few years and present. Such FPs, with their several applications, were recently reviewed by References [3[3][4][9][44],4,9,44], for further readings.
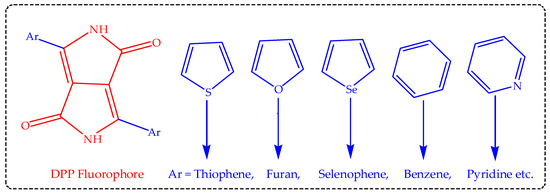
Figure 1. The typical DPP fluorophore’s skeleton structure with some representative various donor units.
1.2. Functionalization of the DPP-Core Unit
Researchers working with this commonly used OD of DPP fluorophore generally adopt two strategic approaches in modifying the DPP core (Figure 2) to develop new derivative with desirable features in mind for different optoelectronics application. These two approaches are (1) functionalizing the DPP core with redox active metals at amide hydrogen (N-H) and (2) functionalization through side-chain engineering at either the (N-H) or the carbonyl center (C=O) of the of the typical DPP core unit. Moreover, when DPP is optimized at the amide position, it mostly generates new derivatives with improved FQY, and when the core unit is functionalized at the 3/6 position, the resulting DPP derivatives displayed improved absorption/emission to NIR region of an electromagnetic spectrum. These modification strategies are all briefly discussed in the following subsections below.
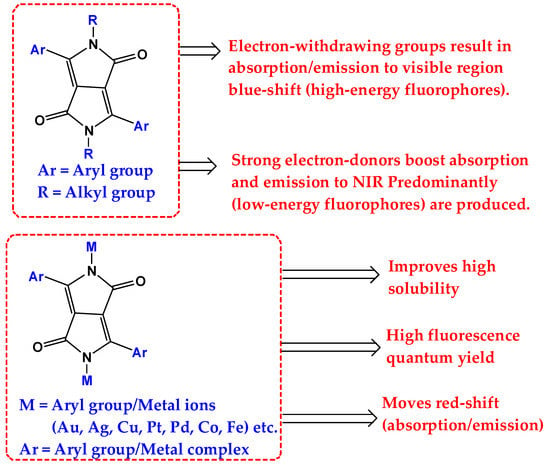
Figure 2. The general DPP core modification strategies using different electron-donating/withdrawing groups at lactam nitrogen and 3/6 positions.
2. Fluorescence Quantum Yields (FQYs) and Emission Properties of DPP FPs
The FQY of materials in other words quantum efficiency is solely the ability of the material’s fluorophore to generate high output power (emitted photons divide by absorbed photons). Moreover, the extent with which the glowing material is able to emit the absorbed light (fluoresce), the better the FQY of that material. A 100% FQY is obtained when the output power = 1, this can only be achieved when the absorbed photons are equal to the emitted photons (1:1 ratio).
Generally, most glowing materials possess a wider excitation and emission spectrum in nature; however, materials with evidently well-defined excitation and emission maxima are crucial and essentially more suitable in fluorescence microscopy like imagine. For this reason, DPP-based FPs are specifically selected owing to the DPPs obviously known very clear distinct absorption and emission wavelengths starting from 400 and 500 nm, respectively [16], in an electromagnetic spectrum for almost all of its derivatives. Furthermore, such DPP FPs are rarely observed (especially in solid-state) among its several derivatives, according to the literature. Table 2, below, summarizes their different photophysical features.
Table 2. Summarized the photophysical features of variously studied fluorescent DPPs.
DPP FPs | Solvent | Abs./Em. (nm) | FQY (%) | (M−1 cm−1) | Stoke’s Shift (cm−1) | FL (ns) | Reference | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
7e (NMe2) | DCM | 684/753 | 3 | 76,800 | 1322 |
[56] |
[45] |
|||||||||||||||||||||||||
7a (H) | DCM | 613/637 | 36 | 44,500 | 600 |
[56] |
[45] |
|||||||||||||||||||||||||
7b (Me) | DCM | 621/646 | 32 | 54,700 | 610 |
[56] |
[45] |
|||||||||||||||||||||||||
7f (CN) | DCM | 622/656 | 32 | 44,800 | 795 |
[56] |
[45] |
|||||||||||||||||||||||||
7c (SMe) | DCM | 627/664 | 14 | 57,000 | 873 |
[56] |
[45] |
|||||||||||||||||||||||||
7d (OMe) | DCM | 631/662 | 22 | 57,100 | 740 |
[56] |
[45] |
|||||||||||||||||||||||||
PDPP-Hex | Toluene | 470/535 | 85 | 12,600 | 0.32 (eV) | 6.8 |
[57] |
[46] |
||||||||||||||||||||||||
TDPP-Hex | Toluene | 510–525/565 | 79 | 27,600 | 0.04 (eV) | 6.2 |
[57] |
[46] |
||||||||||||||||||||||||
SeDPP-Hex | Toluene | 550–562/580 | 66 | 33,600 | 0.07 (eV) | 5.5 |
[57] |
[46] |
||||||||||||||||||||||||
FDPP–QAS | H2O | 534/588 | 55 |
[58] |
[47] |
|||||||||||||||||||||||||||
TDPP–QAS | H2O | 530/595 | 47 |
[58] |
[47] |
|||||||||||||||||||||||||||
SeDPP–QAS | H2O | 537/618 | 42 |
[58] |
[47] |
|||||||||||||||||||||||||||
Phenyl DPP 5 | PBS | 455/515 | 57 | 22,700 | 6.5 |
[59] |
[48] |
|||||||||||||||||||||||||
Thienyl DPP 6 | PBS | 530/596 | 49 | 30,700 | 4.4 |
[59] |
[48] |
|||||||||||||||||||||||||
Optimized phenyl DPP 7 | PBS | H 2O | 456/548 | 456/548 | 76 | 94 | 14,700 | 14,700 | 1350 | 6.5 | 6.8 |
[59] |
[48] |
|||||||||||||||||||
Optimized thienyl DPP 8 | PBS | H 2O | 527/594 | 527/594 | 56 | 80 | 18,100 | 18,100 | 4.8 | 5.0 |
[59] |
[48] |
||||||||||||||||||||
DPP 1 | DCM | PBS | 609/636 | 576/635 | 48 | 2.0 | 43 | 42 | 27 | 59 |
[42] |
|||||||||||||||||||||
DPP 2 | DCM | PBS | 608/635 | 575/637 | 42 | 1.0 | 48 | 47 | 27 | 62 |
[42] |
|||||||||||||||||||||
PhDPP | MeCN | H 2O | 471/556 | 481/604 | 20 | 8 | 3246 | 4234 | 6.21 | 2.33 |
[15] |
|||||||||||||||||||||
PhODPP | DMSO, H2O | 475/550 | 481/599 | 95 | 56 | 2871 | 4096 | 6.38 | 3.62 |
[15] |
||||||||||||||||||||||
M1 (OMe) | THF | 501/646 | 1.1 | 0.32 |
[60] |
[49] |
||||||||||||||||||||||||||
M2 (OMe) | THF | 514/625 | 1.3 | 0.53 |
[60] |
[49] |
||||||||||||||||||||||||||
D1 no OMe | THF | 495/611 | 46.2 | 2.89 |
[60] |
[49] |
||||||||||||||||||||||||||
D2 no OMe | THF | 506/595 | 67.5 | 3.10 |
[60] |
[49] |
||||||||||||||||||||||||||
T2 (TPA) | THF | 503/590 | 71 | 30,000 | 87 |
[61] |
[50] |
|||||||||||||||||||||||||
D2 (TPA) | THF | 504/602 | 68 | 42,200 | 98 |
[61] |
[50] |
|||||||||||||||||||||||||
M2 (TPA) | THF | 513/625 | 2 | 50,500 | 112 |
[61] |
[50] |
FPs, fluorescent probes; FL, fluorescence lifetime.
As seen above, Table 2 summarizes the different photophysical features of some representative DPP FPs recently published, in that almost all of these probes displayed red-shifted absorption and emission in NIR, presenting appreciable FQY, molar absorptivity, Stokes’s shift with good results for AIE, solid-state emission and biological applications. Precisely, their FQYs were quenched in some cases, which was likely due to solvent effect (polarity) or substituent effect incorporated with the DPP fluorophore being it electron-donating or electron-withdrawing groups. In essence, it is obviously known that donating groups result in generating the resulting fluorophores to predominantly absorb/emit in red-shift region; however, this depends on the donor group’s interaction strength with the material’s fluorophore through intramolecular charge transfer (ICT). However, withdrawing groups generally leads to developing fluorophores predominantly moves to blue-shift region low wavelength (high-energy) again, which relies on the withdrawing group strength and its interaction with the material’s fluorophore under investigation via ICT. So in a nut shell, strong donating groups are essentially preferred when optimizing DPP fluorophore especially for biological applications, as such optimized fluorophores are significantly useful in SC, owing to their low-energy, which is very vital for fluorescence studies (bio-imaging, therapy, etc.), due to their deeper tissue penetration ability with nearly zero cytotoxicity. However, fluorescence turn-on/quenching events do happen particularly for applications in biological systems while interacting with target species; an example from Table 2 is briefly discussed, herein, under the molecular/bio-imaging probes section.
Series of DPP-based FPs coded (7a–f), bearing peripheral substituents of (H, Me, CN, SMe, OMe and NMe2), were synthesized, purposely, to study their photophysical effects both for electron-donating and withdrawing substituents to the central DPP for different chromophores independently (Figure 3)56 [56][45], as very little is known from the literature for this strategic approach. Only derivative 7e having strong electron-donating NMe2 was strongly quenched by displaying FQY of 3% with absorption and emission peaks nearing the infrared region at 684 and 753 nm, respectively, tested in DCM solution; however, this nonstructural emissive band exhibited the biggest Stoke’s shift of 1322 cm−1 among them. The bathochromically shifted absorption band along with observed FQ of 7e results from the planarity via extended π–π conjugation system of the bithiophene motifs towards the acceptor unit (the central DPP). Consequently, FQY for 7a (H) was 36%, whereas both 7b and 7f with Me and CN, respectively, show 32%, and were also red-shifted to NIR with absorption and emission bands range of 613 to 656 nm.
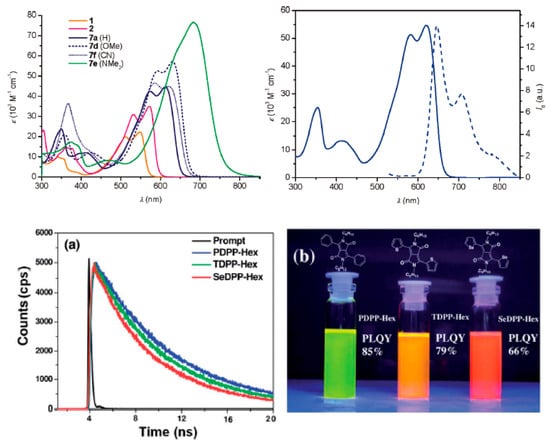
Figure 3. Row-1: left, the DPP derivatives 1, 2 and 7a,d–f UV–vis spectral absorption in DCM (2 × 10−5 M) at 20 °C. 7b (Me) and 7c (SMe) shows very clear comparable spectral features with 7d (OMe); Right-hand side, spectral features in DCM Absorption (solid line) with emission (dashed line, excited at 520 nm) of DPP 7b (Me) at 20 °C. Copyright, 2011, reproduced with permission from Reference [56][45]. Row-2: (a) fluorescence decay pattern of varied DPPs having dissimilar donor units. (b) Pictures showing varied radiating behaviors of three differently prepared DPPs illuminated under (~365 nm) UV light. Copyright, 2014, reproduced with permission from Reference [57][46].
Later on, Dhar et al. reported an observed decreased in FQY of 85%, 79% and 66% for phenyl, thiophene and selenophene DPPs, showing green, orange and red colors (different wavelengths), respectively (Figure 3a,b)57; such declines were ascribed to the effective heavier nature of sulfur and selenium atoms which favors inter-system crossing (ISC), and similarly their fluorescence lifetime (FL) decreased in the same way with decayed lifetime from 6.8, to 6.2 and 5.5 ns, respectively, for phenyl, thiophene and selenium derivatives [57][46]. Their absorption spectra show dual emission bands at 510 and 525 nm for thiophene derivative and then 550 and 562 nm for SeDPP-Hex, but PDPP-Hex displayed lowest energy band at 470 nm. Their fluorescence spectra displayed a bathochromic red-shifted emissive bands of 535, 565 and 580 nm for phenyl, thiophene and selenophene, correspondingly, with displayed dual-band vibronic emission. Another quenched in FQYs was also reported by Wang et al. with DPPs bearing quaternary ammonium salts (QAS) used for investigating their levelling efficiency in copper electroplating technology. The coded FPs are; FDPP–QAS (55%) a furan-based, TDPP–QAS (47%) a thiophene-based and for selenophene-based is SeDPP–QAS (42%) shows a correspondingly similar gradual decline in their observed FQY which looks bright for these dyes after illuminated with UV light at 365 nm [58][47].
In another development later in 2015, authors of Reference [59][48] demonstrated an intensely slight bathochromic shift at 548 and 553 nm, respectively, with FQYs of 57% and 49% for a phenyl and thienyl DPP derivatives coded 5 and 6 having carboxylic acid at their terminal ends. These materials happen to reveal an intense absorption at 460 nm with emission at 530 nm in PBS solution of pH = 7.4 for both DPP–phenyl and DPP–thienyl respectively. Further modification of these dyes with a taurine-like disulfonated substituent, phenyl and thienyl derivatives coded 7 and 8 are obtained Table 2 above, and such optimization boosted their FQYs to 76% and 56% respectively. Upon binding these dyes with BSA protein, the spectral pattern of bound-dyes (BSA-7 and BSA-8), compared to unbound-dyes, remains the same with very high FQYs implying a fluorescence turn-on event, which are slightly lower by only 20% in relation to the freely unbound-dyes. However, the DPP–phenyl exhibited a bigger Stoke’s shift of 1350 cm−1 with an evident vibronic band sequence vividly observed in contrast to the DPP–thienyl. Their FL was found to be (4.5 to 6.5 ns) similar with most commonly soluble ODs. Tang et al. (2017) presents other FPs coded DPP1 and DPP2 incorporated with heterocyclic aryl amine flanked to the DPP, with each bearing another free carboxylic acid moiety for conjugation with biomolecules. Absorption spectrum of these dyes shows a vibronic with shoulder peaks around 565 and 578 nm in DCM, DMSO and MeCN, nonetheless, their electronic absorption in PBS were slightly blue-shifted to 575 nm and conversely, their emission spectra lies mainly in far-red region. These dyes experience a decreased FQYs in different organic solvents, were 0.48 and 0.42 (DCM), 0.38 and 0.26 (DMSO), and 0.13 and 0.16 (MeCN), displayed for DPP1 and DPP2, respectively. Solvent polarity effect leads to the observed FQY decline from DCM to DMSO through (MeCN) [42]. Such decline in FQY was found even more pronounced in PBS pH 7.4 with 0.01 for DPP1 and 0.02 for DPP2 dyes, probably this is favored due to the hydrogen-bonding, as such leads to the generation of an electron-accepting water cages which might be possible for heterocyclic amines. However, upon conjugation with BSA protein, both dyes displayed a strongly enhanced fluorescence emission especially with DPP2 an 18-fold FQY increased a protein-induced fluorescence enhancement (PIFE), implying protein hydrophobicity to have greatly restricted bound-BSA of DPPs to vibrate. These findings contradict BSA-7 and BSA-8 bio-conjugation previously discussed here, in this section [59][48].
3. Solid-State Fluorescence of DPPs with Their Aggregation-Induced Emission Enhancement (AIEE/AIE) in Solution and Water
3.1. Solid-State Emission
Fluorescence in solid-state is of paramount importance in organic optoelectronics science, this is because organic fluorophores are an integral components of organic light emitting electronic devices like (OLEDs), solar cells [65][51], etc., as such applications needs such fluorophores to be fabricated into compacted solid films [66][52]. Therefore, the possibility of organic fluorophores to come together and aggregate to form solid films is the fundamental basis of aggregation-caused quenching (ACQ) phenomena, which significantly limits their biological applications like bio-imaging, as well as their organic electronics technological advancement.
4. Probes for Molecular/Bio-Imaging Applications
Biomolecules are the famously known endogenous biomaterials secreted internally within an organism’s biological system. Moreover, the biomaterials consist of proteins, carbohydrates, lipids and nucleic acids, in addition to primary and secondary metabolites along with other natural products. Generally, biomolecules are organic materials composed of oxygen, carbon, hydrogen and nitrogen as their major building block constituting about 96% weight of human body with trace amounts of other components, like biometals, in it. Biomolecules recognition is necessary because their chemical composition, biochemistry and interactions in the biological system reflect the physical and physiological functions (well-being or abnormality) scientifically. Therefore, such integral components are being detected and monitored concurrently, using different strategies in healthcare units.
Two-photon fluorescence microscopy (2PFM) presents a significantly deep tissue penetration (>500 µm) [16], and hence it serves as a promising platform for biomolecules recognition, bio-imaging, therapy, etc., due to its noninvasiveness in biosensing of living cells and tissues. This results from the recently explored numerous reports of luminescent conjugated DPPs by studying their two-photon excitation/absorption behaviors in the biological system. The conjugated framework of this strongly acceptor core unit of DPP incorporating various donor groups were explored and have shown advancement in realizing an efficiently two-photon FPs. As a result, two-photon DPP FPs demonstrated valuable TPA cross-sectional values ranging (500–3000 GM) and above, hence indicating their potent deep tissue penetrability for imaging application to detect cancer/tumor diseases, and in some cases, they were even reported to have destroyed such cells.
References
- Dai, J.; Ma, C.; Zhang, P.; Fu, Y.; Shen, B. Recent progress in the development of fluorescent probes for detection of biothiols. Dye. Pigment. 2020, 177, 108321.
- Jiang, X.; Wang, L.; Tang, H.; Cao, D.; Chen, W. Diketopyrrolopyrrole: An emerging phototherapy agent in fighting cancer. Dye. Pigment. 2020, 181, 108599.
- Li, W.; Wang, L.; Tang, H.; Cao, D. Diketopyrrolopyrrole-based fluorescent probes for detection and bioimaging: Current progresses and perspectives. Dye. Pigment. 2019, 162, 934–950.
- Li, J.; Pu, K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem. Soc. Rev. 2019, 48, 38–71.
- Chakali, M.; Mandal, H.; Venkatesan, M.; Dyaga, B.; Rao, V.J.; Bangal, P.R. Charge Separation and Singlet Fission in Covalently Linked Diketopyrrolopyrrole Derivatives and Triphenylamine Triad in Solution. J. Photochem. Photobiol. A Chem. 2020, 406, 113017.
- Zhou, K.; Dai, K.; Liu, C.; Shen, C. Flexible conductive polymer composites for smart wearable strain sensors. SmartMat 2020, 1, e1010.
- Antón-García, D.; Warnan, J.; Reisner, E. A diketopyrrolopyrrole dye-based dyad on a porous TiO2 photoanode for solar-driven water oxidation. Chem. Sci. 2020, 11, 12769–12776.
- Hwang, T.G.; Kim, G.-Y.; Han, J.-I.; Kim, S.; Kim, J.P. Enhancement of Lipid Productivity of Chlorella sp. Using Light-Converting Red Fluorescent Films Based on Aggregation-Induced Emission. ACS Sustain. Chem. Eng. 2020, 8, 15888–15897.
- Liu, Q.; Bottle, S.E.; Sonar, P. Developments of Diketopyrrolopyrrole-Dye-Based Organic Semiconductors for a Wide Range of Applications in Electronics. Adv. Mater. 2020, 32, 1903882.
- Zhang, R.; Sun, M.; Wang, X.; Yan, H.; Zhang, G.; Zhang, Q. Polymerizations of Diketopyrrolopyrrole-Type Dyes in Unconventional Orientation. ACS Appl. Polym. Mater. 2020, 2, 5698–5704.
- Wang, Y.; Yang, J.; Gong, Y.; Fang, M.; Li, Z.; Tang, B.Z. Host–guest materials with room temperature phosphorescence: Tunable emission color and thermal printing patterns. SmartMat 2020, 1, e1006.
- Wang, Q.; Xia, B.; Xu, J.; Niu, X.; Cai, J.; Shen, Q.; Wang, W.; Huang, W.; Fan, Q. Biocompatible small organic molecule phototheranostics for NIR-II fluorescence/photoacoustic imaging and simultaneous photodynamic/photothermal combination therapy. Mater. Chem. Front. 2019, 3, 650–655.
- Ghosh, S.; Shankar, S.; Philips, D.S.; Ajayaghosh, A. Diketopyrrolopyrrole-based functional supramolecular polymers: Next-generation materials for optoelectronic applications. Mater. Today Chem. 2020, 16, 100242.
- Chiminazzo, A.; Borsato, G.; Favero, A.; Fabbro, C.; McKenna, C.E.; Dalle Carbonare, L.G.; Valenti, M.T.; Fabris, F.; Scarso, A. Diketopyrrolopyrrole Bis-Phosphonate Conjugate: A New Fluorescent Probe for In Vitro Bone Imaging. Chem. A Eur. J. 2019, 25, 3617–3626.
- Abelha, T.F.; Morris, G.; Lima, S.M.; Andrade, L.H.; McLean, A.J.; Alexander, C.; Calvo-Castro, J.; McHugh, C.J. Development of a Neutral Diketopyrrolopyrrole Phosphine Oxide for the Selective Bioimaging of Mitochondria at the Nanomolar Level. Chem. A Eur. J. 2020.
- Kaur, M.; Choi, D.H. Diketopyrrolopyrrole: Brilliant red pigment dye-based fluorescent probes and their applications. Chem. Soc. Rev. 2015, 44, 58–77.
- Sun, X.-Y.; Liu, T.; Sun, J.; Wang, X.-J. Synthesis and application of coumarin fluorescence probes. Rsc Adv. 2020, 10, 10826–10847.
- Rajasekar, M. Recent development in fluorescein derivatives. J. Mol. Struct. 2020, 1224, 129085.
- Fu, Z.-H.; Han, X.; Shao, Y.; Fang, J.; Zhang, Z.-H.; Wang, Y.-W.; Peng, Y. Fluorescein-based chromogenic and ratiometric fluorescence probe for highly selective detection of cysteine and its application in bioimaging. Anal. Chem. 2017, 89, 1937–1944.
- Zhang, Q.; Wong, K.M.-C. Photophysical, ion-sensing and biological properties of rhodamine-containing transition metal complexes. Coord. Chem. Rev. 2020, 416, 213336.
- Fischer, G.M.; Daltrozzo, E.; Zumbusch, A. Selective NIR chromophores: Bis (pyrrolopyrrole) cyanines. Angew. Chem. Int. Ed. 2011, 50, 1406–1409.
- Fischer, G.M.; Isomäki-Krondahl, M.; Göttker-Schnetmann, I.; Daltrozzo, E.; Zumbusch, A. Pyrrolopyrrole cyanine dyes: A new class of near-infrared dyes and fluorophores. Chem. A Eur. J. 2009, 15, 4857–4864.
- Wiktorowski, S.; Rosazza, C.; Winterhalder, M.J.; Daltrozzo, E.; Zumbusch, A. Water-soluble pyrrolopyrrole cyanine (PPCy) NIR fluorophores. Chem. Commun. 2014, 50, 4755–4758.
- Sun, C.; Du, W.; Wang, B.; Dong, B.; Wang, B. Research progress of near-infrared fluorescence probes based on indole heptamethine cyanine dyes in vivo and in vitro. Bmc Chem. 2020, 14, 1–28.
- Li, Y.; Zhou, Y.; Yue, X.; Dai, Z. Cyanine Conjugate-Based Biomedical Imaging Probes. Adv. Healthc. Mater. 2020, 9, 2001327.
- Shimizu, S.; Iino, T.; Araki, Y.; Kobayashi, N. Pyrrolopyrrole aza-BODIPY analogues: A facile synthesis and intense fluorescence. Chem. Commun. 2013, 49, 1621–1623.
- Alnoman, R.B.; Parveen, S.; Hagar, M.; Ahmed, H.A.; Knight, J.G. A new chiral boron-dipyrromethene (BODIPY)-based fluorescent probe: Molecular docking, DFT, antibacterial and antioxidant approaches. J. Biomol. Struct. Dyn. 2020, 38, 5429–5442.
- Wang, J.; Xu, W.; Yang, Z.; Yan, Y.; Xie, X.; Qu, N.; Wang, Y.; Wang, C.; Hua, J. New diketopyrrolopyrrole-based ratiometric fluorescent probe for intracellular esterase detection and discrimination of live and dead cells in different fluorescence channels. Acs Appl. Mater. Interfaces 2018, 10, 31088–31095.
- Du, W.; Ohayon, D.; Combe, C.; Mottier, L.; Maria, I.P.; Ashraf, R.S.; Fiumelli, H.; Inal, S.; McCulloch, I. Improving the compatibility of diketopyrrolopyrrole semiconducting polymers for biological interfacing by lysine attachment. Chem. Mater. 2018, 30, 6164–6172.
- Ni, Z.; Dong, H.; Wang, H.; Ding, S.; Zou, Y.; Zhao, Q.; Zhen, Y.; Liu, F.; Jiang, L.; Hu, W. Quinoline-Flanked Diketopyrrolopyrrole Copolymers Breaking through Electron Mobility over 6 cm2 V−1 s−1 in Flexible Thin Film Devices. Adv. Mater. 2018, 30, 1704843.
- Wang, Z.; Liu, Z.; Ning, L.; Xiao, M.; Yi, Y.; Cai, Z.; Sadhanala, A.; Zhang, G.; Chen, W.; Sirringhaus, H. Charge mobility enhancement for conjugated DPP-selenophene polymer by simply replacing one bulky branching alkyl chain with linear one at each DPP unit. Chem. Mater. 2018, 30, 3090–3100.
- Chandran, D.; Lee, K.-S. Diketopyrrolopyrrole: A versatile building block for organic photovoltaic materials. Macromol. Res. 2013, 21, 272–283.
- Patil, H.; Gupta, A.; Bilic, A.; Bhosale, S.V.; Bhosale, S.V. A solution-processable electron acceptor based on diketopyrrolopyrrole and naphthalenediimide motifs for organic solar cells. Tetrahedron Lett. 2014, 55, 4430–4432.
- Patil, Y.; Misra, R. Rational molecular design towards NIR absorption: Efficient diketopyrrolopyrrole derivatives for organic solar cells and photothermal therapy. J. Mater. Chem. C 2019, 7, 13020–13031.
- Yang, X.; Cui, Y.; Li, Y.; Zheng, L.; Xie, L.; Ning, R.; Liu, Z.; Lu, J.; Zhang, G.; Liu, C. A new diketopyrrolopyrrole-based probe for sensitive and selective detection of sulfite in aqueous solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 1055–1060.
- Schutting, S.; Borisov, S.M.; Klimant, I. Diketo-pyrrolo-pyrrole dyes as new colorimetric and fluorescent pH indicators for optical carbon dioxide sensors. Anal. Chem. 2013, 85, 3271–3279.
- Kaur, M.; Choi, D.H. Dual channel receptor based on diketopyrrolopyrrole alkyne conjugate for detection of Hg2+/Cu2+ by “naked eye” and fluorescence. Sens. Actuators B Chem. 2014, 190, 542–548.
- Aigner, D.; Ungerböck, B.; Mayr, T.; Saf, R.; Klimant, I.; Borisov, S.M. Fluorescent materials for pH sensing and imaging based on novel 1, 4-diketopyrrolo-[3, 4-c] pyrrole dyes. J. Mater. Chem. C 2013, 1, 5685–5693.
- Fischer, G.M.; Ehlers, A.P.; Zumbusch, A.; Daltrozzo, E. Near-infrared dyes and fluorophores based on diketopyrrolopyrroles. Angew. Chem. Int. Ed. 2007, 46, 3750–3753.
- Qu, Y.; Wu, Y.; Gao, Y.; Qu, S.; Yang, L.; Hua, J. Diketopyrrolopyrrole-based fluorescent conjugated polymer for application of sensing fluoride ion and bioimaging. Sens. Actuators B Chem. 2014, 197, 13–19.
- Grzybowski, M.; Glodkowska-Mrowka, E.; Hugues, V.; Brutkowski, W.; Blanchard-Desce, M.; Gryko, D.T. Polar Diketopyrrolopyrrole-Imidazolium Salts as Selective Probes for Staining Mitochondria in Two-Photon Fluorescence Microscopy. Chem. A Eur. J. 2015, 21, 9101–9110.
- Tang, S.; Zadeh, E.H.G.; Kim, B.; Toomey, N.T.; Bondar, M.V.; Belfield, K.D. Protein-induced fluorescence enhancement of two-photon excitable water-soluble diketopyrrolopyrroles. Org. Biomol. Chem. 2017, 15, 6511–6519.
- Ftouni, H.; Bolze, F.D.R.; de Rocquigny, H.; Nicoud, J.-F.O. Functionalized two-photon absorbing diketopyrrolopyrrole-based fluorophores for living cells fluorescent microscopy. Bioconjugate Chem. 2013, 24, 942–950.
- Ren, X.; Yang, F.; Gao, X.; Cheng, S.; Zhang, X.; Dong, H.; Hu, W. Organic Field-Effect Transistor for Energy-Related Applications: Low-Power-Consumption Devices, Near-Infrared Phototransistors, and Organic Thermoelectric Devices. Adv. Energy Mater. 2018, 8, 1801003.
- Bürckstümmer, H.; Weissenstein, A.; Bialas, D.; Würthner, F. Synthesis and characterization of optical and redox properties of bithiophene-functionalized diketopyrrolopyrrole chromophores. J. Org. Chem. 2011, 76, 2426–2432.
- Dhar, J.; Venkatramaiah, N.; Anitha, A.; Patil, S. Photophysical, electrochemical and solid state properties of diketopyrrolopyrrole based molecular materials: Importance of the donor group. J. Mater. Chem. C 2014, 2, 3457–3466.
- Wang, K.; Feng, J.; Xu, J.; Li, J.; Mai, M.; Wang, X.; Wang, L. Engineering aromatic heterocycle strategy: Improving copper electrodeposition performance via tuning the bandgap of diketopyrrolopyrrole-based leveler. Tetrahedron 2020, 76, 130882.
- Heyer, E.; Lory, P.; Leprince, J.; Moreau, M.; Romieu, A.; Guardigli, M.; Roda, A.; Ziessel, R. Highly Fluorescent and Water-Soluble Diketopyrrolopyrrole Dyes for Bioconjugation. Angew. Chem. 2015, 127, 3038–3042.
- Hwang, T.G.; Han, G.R.; Lee, J.M.; Lee, J.W.; Kim, H.M.; Hwang, D.; Kim, S.K.; Kim, J.P. Fluorescence Quenching of 4, 4′-Dimethoxytriphenylamine-Substituted Diketopyrrolopyrrole via Intramolecular Photoinduced Electron Transfer. J. Phys. Chem. C 2019, 123, 24263–24274.
- Hwang, T.G.; Kim, J.Y.; Namgoong, J.W.; Lee, J.M.; Yuk, S.B.; Kim, S.H.; Kim, J.P. Aggregation induced emission of diketopyrrolopyrrole (DPP) derivatives for highly fluorescent red films. Photochem. Photobiol. Sci. 2019, 18, 1064–1074.
- Yao, Y.; Chen, Y.; Wang, H.; Samorì, P. Organic photodetectors based on supramolecular nanostructures. SmartMat 2020, 1.
- Graziano, G. Solid-state fluorescence: Under pressure. Nat. Rev. Chem. 2017, 1.