Exosomes are nano-vesicle-shaped particles secreted by various cells, including cancer cells. Many studies have focused on the bioactive molecules that they export as exosomal cargo. These molecules can function as biomarkers in diagnosis or play a relevant role in modulating the immune system and in promoting apoptosis, cancer development and progression.
- exosomes
- oncosomes
- cancer
- exosomal cancer evolution
- exosomal cancer therapy
- exosomal cancer cell reprogramming
1. Introduction
Exosomes are nano-vesicle-shaped particles secreted under physiological and pathological conditions by various cells, including cancer cells. They export nucleic acids, proteins and lipids that target close or distant cells, thus regulating multiple cellular processes. Cancer research has demonstrated that these particles provide biomarkers for diagnosis [1[1][2][3],2,3], induce apoptosis, modulate the immune system and play a relevant role in promoting cancer development, progression and metastasis. Moreover, specific exosomes are potentially helpful for cancer therapy by various modalities, including re-differentiation of malignant cells. This review first describes the principal morphological-functional characteristics of exosomes. Then, it discusses how tumour-derived exosomes induce cancer evolution and metastasis by affecting the tumour microenvironment (TME) and immune system. Then, the potentially helpful role of exosomes for cancer therapy is considered. Particularly, an increased consensus has been gained by the use of exosomes as biological reprogrammers of cancer cells. Elucidation of the molecular mechanisms of TME reprogramming allows more general conclusions to be drawn for the development of new interventions that are particularly focused on their use as epigenetic reprogramming therapy for malignant cells.
2. The Exosomes
The biogenesis of exosomes and their principal characteristics, well described in recent review articles [4[4][5][6][7],5,6,7], are briefly considered (Figure 1A,B).
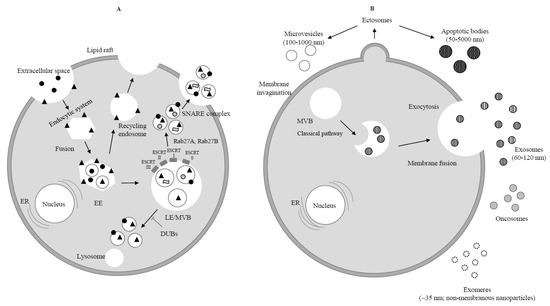
Figure 1. Biogenesis of exosomes. (A) Endocytosis begins at the cytoplasmic membrane. Early endosomes (EEs) form by the fusion of uncoated endocytic vesicles. EEs either return to the plasma membrane (recycling endosome) or convert into late endosomes/multivesicular bodies (LEs/MVBs). Protein sorting of ILVs (intraluminal vesicles) occurs through endosomal sorting complexes required for transport (ESCRT) dependent or independent mechanism. ESCRT 0, ESCRT I, ESCRT II, ESCRT III are four components of ESCRT machinery that ubiquitinate the substrates on the part of the inward budding endosomal membrane. ILVs are ready to be degraded by lysosome or rescued by de-ubiquitinating enzymes (DUBs) through Rab GTPases (Rab27A and Rab27B) which allow the MVBs to move to the cell periphery. Finally MVBs through the SNARE complex fuse with the plasma membrane and lead to exocytosis (release of ILVs as exosomes to the extracellular space).  Extracellular protein;
Extracellular protein;  Receptor;
Receptor;  Lipid;
Lipid; 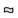 RNA. (B) Ectosomes derive from the plasma membrane by direct gemmation. Large oncosomes, as microvesicles originate from plasma membrane [8]. Exosomes are derived and secreted through a “classical” pathway. ER = endoplasmic reticulum.
RNA. (B) Ectosomes derive from the plasma membrane by direct gemmation. Large oncosomes, as microvesicles originate from plasma membrane [8]. Exosomes are derived and secreted through a “classical” pathway. ER = endoplasmic reticulum.  Microvesicle;
Microvesicle;  Apoptotic bodies;
Apoptotic bodies;  Exosome;
Exosome;  Exomere;
Exomere;  Oncosome.
Oncosome.
3. The Role of Exosomes in Cancer
An emerging concept is that cancer cells can release different subtypes of EVs together with those derived from normal cells [8]. Very large EVs (1–10 μm) from cancer cells, named “large oncosomes” or simply “oncosomes” and derived from the shedding of ILVs, have also been described recently [51][9]. These large vesicles mainly have aberrant expression of some different oncoproteins, such as myristoylated Akt (MyrAkt)1, heparin-binding epidermal growth factor (HB-EGF) and caveolin-1, suggesting that oncosomes play a key role in cancer cells [52][10]. Tumour-derived EVs contain many proteinic enzymes involved in glucose, glutamine and amino acid metabolism. This suggests that these EVs play a relevant role in governing the metabolism of cancer cells. [53][11]. Tumour-derived EVs also provide instructions for extracellular communication, cellular differentiation and migration [54][12]. The possible release of cell-free nucleic acids (cfNAs) into blood by exosomes has suggested their relationship [55[13][14],56], while genetic information transferred from the exosomes to the host cells could transform normal cells by inducing oncogenic mutations and could be involved in promoting genetic instability in target cells [57][15]. Exosomes contain double-stranded DNA (dsDNA) from the parent cell, and exosomal DNA in tumour-derived exosomes exerts important translational value by acting as a circulating biomarker in the early detection of cancer and metastasis [58][16]. In addition, lncRNAs are exchanged between gastric cancer cells through exosomes, triggering cancer progression [59][17]. Nucleic acids in exosomes can be studied by nano-particle tracking analysis (ZetaView), Western blotting, transmission electron microscopy and other techniques [60][18]. Often, only partial correspondence of tissue profiles with tumour exosome profiles, mainly referring to small RNAs, has been reported [61,62][19][20]. The exosomal capability of transferring information involves, in addition to cancer cells, the close and distant TME [63][21]. During cancer development, oncosomes from brain cancer cells can transfer the oncogenic receptor EGFRvIII to other cancer cells that do not have this receptor [64][22]. Oncosomes transfer transforming growth factor beta (TGF-β) from cancers to normal fibroblasts, thus promoting myofibroblast differentiation [65][23]. Conversely, oncosomes from cancer-associated fibroblasts (CAF) inhibit mitochondrial oxidative phosphorylation, thus regulating the metabolism of cancer cells [66][24]. Exosomes secreted from breast or pancreatic cancers contribute to the formation of metastatic niche carrying telomerase [67][25] or macrophage migration inhibitory factor (MIF) [68][26] to the TME. In a study, exosomes carrying TNF-related apoptosis-inducing ligand (TRAIL) re-established apoptosis at the tumour site in vitro and in a preclinical mouse model [69][27]. The relationships of tumour-derived exosomes with cancer evolution and the immune system are discussed in more detail in the following sections.
4. Use of Exosomes in Cancer Therapy
Having described the role that tumour-derived exosomes play as carriers of specific substances that can spread tumours in an organism, we would now like to illustrate in the next paragraphs the use of exosomes as carriers of many substances with anticancer properties.
4.1. Exosomes as Carriers of Natural Substances or Chemotherapeutical Drugs with Anticancer Properties
Exosomes can function as drug delivery carriers in cancer therapy [146][28]. Taking into account that exosomes are very small particles, they can penetrate the cell membrane easily. Many studies about exosomes as drug delivery systems have been conducted, and their high specificity in targeting cancer cells has been widely reported [147][29]. In an experimental study, direct and specific targeting of oncogenic KRAS by engineered exosomes from normal fibroblast-like mesenchymal cells was described [148][30]. In 2016, researchers used exosomes into which they introduced curcumin to treat cancer patients [149][31]. Curcumin is a known biological substance with recognized anticancer properties. Their studies proved that exosomes can transfer curcumin into H1299 cells, causing TCF21 up-regulation with anticancer effects [149][31], while curcumin alone is inefficient because it lacks bioavailability [150,151][32][33]. In addition, some studies have been conducted on the ability of exosomes to transport chemotherapeutic agents. For example, in 2014, some researchers tested the capacity of exosomes as a drug delivery system to transfer chemotherapeutic agents such as doxorubicin [152,153][34][35]. The results showed that when administered in exosomes, doxorubicin inhibited cell proliferation in BALB/c nude mice. Exosomes were obtained by mouse immature dendritic cells (imDCs) to significantly decrease the side effects and immunogenicity. An exosomal membrane protein (Lamp2b) fused to αv integrin-specific iRGD peptide (CRGDKGPDC) was incorporated into imDCs by engineering to favour tumour targeting [154][36]. Electroporation allowed loading of these purified exosomes with doxorubicin, with an encapsulation efficiency of up to 20%. These exosomes, after intravenous injection, greatly inhibited tumour growth, and this treatment was well tolerated. In fact, intravenously injected targeted exosomes showed efficient doxorubicin delivery leading to greater inhibition of various tumour cell lines than doxorubicin alone. In addition, low toxicity was demonstrated when doxorubicin was transferred using exosomes in comparison with the use of doxorubicin alone. In another experimental investigation [155][37], a potent chemotherapeutic agent, paclitaxel (PTX), to treat MDR cancer was evaluated. Different methods of loading exosomes released by macrophages with PTX (exoPTX) that showed high loading efficiency and drug release were developed. Interestingly, incorporation of PTX into exosomes enhanced cytotoxicity more than 50 times in drug-resistant MDCKMDR1 (Pgp+) cells. Moreover, other studies demonstrated a relevant co-localization of airway-delivered exosomes with cancer cells and a strong anticancer activity in a model of murine Lewis lung carcinoma pulmonary metastases. Authors concluded that exoPTX can deliver various chemotherapeutics to treat drug-resistant cancers. It is possible to conclude that exosomes possess the capacity to act as a delivery system of chemotherapeutic agents and that they can increase therapeutic effects and reduce side effects in comparison with the use of the anticancer drug alone.
4.2. Exosomes as Carriers of Boron Neutron Capture Therapy (BNCT)
BNCT joins selective accumulation of 10B carriers in tumour tissue with neutron irradiation. So far, more often, BNCT has been investigated in experimental clinical studies for recurrent malignant gliomas, head and neck cancers as well as multiple lung metastases using neutron beams derived from research reactors [158,159][38][39]. Phenylboronic acid-functionalized nanocarriers for specific targeting to sialic acid groups over-expressed on tumour cells appear to be a very promising mechanism [160][40]. Currently, two low-molecular-weight boron-containing drugs are being used clinically, boronophenylalanine (BPA) and sodium borocaptate (BSH). However, in the last years, because of some limitations, great effort has been made to develop nanoscaled boron-containing delivery agents with more favourable biodistribution and uptake for clinical use. These potential nanocarriers include exosomes in addition to other nanoparticles of various types [161][41].
4.3. Exosomes as Anticancer Vaccines for Immunotherapy
In recent years, exosomes also have been experimentally employed as a cancer immunotherapy treatment [156,157][42][43]. Particularly, they have been proposed as anticancer vaccines due to their capacity to carry antigens and MHC-peptide complexes and to promote helper T-cell immune responses. In a study [162][44] conducted in a B16 melanoma model, vesicles released as exosomes or microvesicles (MV) and alternatively as apoptotic blebs or vesicles (ApoV) from the plasma membrane of apoptotic cancer cells were characterized. Distinct types of surface and cytoplasmic molecules (tetraspanins, integrins, heat shock proteins and histones) were expressed in the different vesicle types. Interestingly, mice immunized with antigen-pulsed ApoV and challenged with B16 tumours showed significantly longer tumour-free survival than mice immunized with ovalbumin-pulsed exosome vaccine or ovalbumin-pulsed microvesicle vaccine. In a phase I clinical trial, ascites-derived exosomes (Aex) in combination with the granulocyte-macrophage colony-stimulating factor (GM-CSF) were used in the immunotherapy of colorectal cancer (CRC). Overall, 40 patients with advanced CRC were recruited to the study and randomly assigned to treatments with Aex alone or Aex plus GM-CSF. Both therapies were safe and well tolerated; however, Aex plus GM-CSF but not Aex alone induced a tumour-specific antitumour cytotoxic T-lymphocyte (CTL) response [163][45].
4.4. Exosomes as Biological Reprogrammers for Cancer Treatment
The composition of exosomes has been clarified in the last years and, as previously reported, many different proteins have been identified inside exosomes. They carry specific nucleic acids, such as mRNA, that have been shown to induce phenotypic changes in recipient cells. Exosomes from different stem cells and from stem cells at different developmental stages may carry specific information. Exosomes contain proteins and nucleic acids that directly reflect the metabolic state of the cells from which they originate. Exosomes can regulate multiple genes in an epigenetic manner and are capable of producing multiple signal transduction cascades. Communication depends on specific crosstalk between exosomes and the target cells. This paracrine mechanism can mediate cell-to-cell communication via direct receptor stimulation of target cells and the horizontal transfer of genetic material to cells prone to avidly receive this information. Using exosomes for cancer therapy has many potential benefits [164][46]. It was also demonstrated that exosomes secreted by mesenchymal stem cells (MSC) have the ability to control tumour growth due to the specific substances they transfer. Exosomes derived from MSC contain multiple cargoes and proteins that may control different relevant metabolic pathways of malignant cells [165][47]. In 2012, researchers evaluated if exosomes from MSC of human bone marrow can inhibit in vivo and in vitro growth of multiple tumours. The study revealed that exosomes secreted from MSC of human bone marrow inhibited cell cycle progression and induced apoptosis in different cancer cells such as hepatocellular carcinoma, ovarian tumour and Kaposi’s sarcoma [166][48]. Another series of studies emerging in cancer therapy regard the possibility of cancer cell reprogramming. The term “reprogramming” was initially introduced to identify the transformation of a normal adult somatic cell into an embryonic-like stem cell, so-called induced pluripotent stem cells (iPS). The issue of cell reprogramming has now been extended to cancer (stem) cells to define any genetic or epigenetic intervention aimed at inducing differentiation of these cells into a normal phenotype and/or forcing them to become terminally differentiating cells [167][49]. In order to differentiate, tumour cells must go through cell regulation pathways. These pathways can be controlled by multiple cell differentiation factors. For example, exosomes derived from mesenchymal stem cells can function as differentiating factors, because they transfer particular instructions to direct cancer cells toward differentiation or apoptosis. Stem cells are found in and can be isolated from multiple tissues of our bodies, such as adipose tissue, bone marrow and the umbilical cord. Some functions of these cells are to replace damaged cells and to cause differentiation. Very interesting are studies on umbilical cord stem cells (hUCMSCs) derived from Wharton’s jelly due to multiple advantages they possess: in fact, hUCMSCs derived from Warton’s jelly possess a unique transcriptome that shows pro-apoptotic and anticancer properties [168][50]. A study conducted in 2012 demonstrated that human Wharton’s jelly stem cell (hWJSC) extracts significantly attenuated tumour growth in three different cancer cell lines in vitro [169][51] and in another study, it was observed that exosomes from umbilical cord-derived MSC inhibited the growth of breast cancer cells in vitro and in vivo [170][52].
4.5. Stem Cells Differentiation Stage Factors (SCDSFs): Other Reprogramming Factors of Cancers, besides Exosomes
Another interesting path of studies about cancer cell reprogramming regards the research on stem cell differentiation factors and their role in reprogramming cancer (stem) cells. These studies originated on the basis of many previous studies, which demonstrated that cancer development can be prevented during embryonic life due to the factors present during organogenesis, as recorded in previous reviews [171][53]. In fact, studies conducted in our laboratory demonstrated that not only many different human tumour cell lines (glioblastoma multiforme, hepatocellular carcinoma, melanoma, breast cancer and kidney adenocarcinoma) but also non-solid tumours, such as acute lymphoblastic leukaemia, treated with factors taken from zebrafish embryos during organogenesis demonstrated a significant slowdown in their growth only when treated with factors taken at the precise moments of cell differentiation stages [172][54]. The effects of SCDSFs on tumour growth were also observed in vivo after subcutaneous injection of primary Lewis Lung Carcinoma cells into C57BL/6 female syngeneic mice weighing 18–20 g. A highly significant difference was noted (p < 0.001) between treated and control mice in favour of the treated mice [171,173][53][55]. All these experiments confirm the hypothesis that only when the factors taken during the stages of differentiation are used, it is possible to direct tumour cells towards the normal path of differentiation. These factors appear in the phases of cell differentiation, and they are absent in the stages of mere multiplication. It has been demonstrated that SCDSFs taken from zebrafish embryos contain apoptosis-inducing proteins that can act on colon cancer cells [174][56]. In another study, all types of proteins taken from zebrafish embryos after the beginning of stem cell differentiation were identified using liquid chromatography–mass spectrometry (LC-MS/MS) analysis, after the in-gel digestion procedure [173][55]. The identified proteins, which represent 98% of the molecules isolated from SCDSFs (the remaining 2% of the molecules are represented by nucleic acids), included multiple forms of yolk vitellogenin, heat shock protein (e.g., HSP8 and HSP70), which are important for the immune response, and other proteins able to regulate mitochondrial metabolism, etc. These proteins are implicated in many pathways such as signalling in cell cycle regulation, protein trafficking, chaperoning and protein synthesis and degradation. It was confirmed that these proteins have a reprogramming or apoptotic effect on cancer cells because they act to regulate transcriptional activation of p53 [175][57] or a post-translational modification of the protein of the retinoblastoma (pRb), able to reprogram cancer cells [176][58]. Moreover, apoptotic events as well as cell differentiation events were studied, in order to understand the consequences of cell cycle regulation in tumour cells induced by differentiation factors. The analysis was carried out on colon adenocarcinoma cells, showing activation of an apoptotic pathway dependent on p73, as well as an increase in the cell differentiation marker e-cadherin [174][56]. In addition, in order to ascertain if these embryonic factors could synergistically/additively interact with 5-fluorouracil (5-Fu), whole cell-count, flow-cytometry analysis and apoptotic parameters were recorded in human colon cancer cells (Caco2) treated with zebrafish stem cell differentiation stage factors (SCDSF 3 µg/mL) in association or not with 5-Fu in the sub-pharmacological therapeutic range (0.01 mg/mL). Cell proliferation was significantly reduced by SCDSF; meanwhile, SCDSF+5-Fu leads to an almost complete growth inhibition. SCDSF produces a significant apoptotic effect; meanwhile, the association with 5-FU leads to an enhanced additive apoptotic rate at both 24 and 72 h. SCDSF alone and in association with 5-Fu triggers both the extrinsic and the intrinsic apoptotic pathways, activating caspase-8, -3 and -SCDSF and 5-Fu alone exerted opposite effects on Bax and Bcl-xL proteins; meanwhile, SCDSF+5-Fu induced an almost complete suppression of Bcl-xL release and a dramatic increase in the Bax/Bcl-xL ratio [177][59]. These data suggest that zebrafish embryo factors could improve chemotherapy efficacy by reducing anti-apoptotic proteins involved in drug-resistance processes. This information is congruent with other studies, which demonstrate that differentiation factors can possess epigenetic regulators that are able to regulate cancer cells by activating new pathways. In particular in a recent study, it was observed that SCDSFs significantly antagonize proliferation of breast cancer cells, because they not only reduce cell proliferation and enhance apoptosis but also dramatically inhibit both invasiveness and the migrating capabilities of cancer cells, controlling, in this way, tumour spread and metastasis. Moreover, it was demonstrated that SCDSFs are also able to inhibit migration and invasiveness of breast cells in the epithelial-mesenchymal transition phase following TGF-β1 stimulation. The reversion program involves modulation of the E-cadherin/β-catenin pathway, cytoskeleton remodelling with dramatic reduction in vinculin, as well as down-regulation of translationally controlled tumour protein (TCTP) and the concomitant increase in p53 levels [178][60]. In addition, it is noteworthy that SCDSFs have a very important role in cancer treatment when they are transferred by exosomes. In fact, it was demonstrated that SCDSFs contained in exosomes derived from mesenchymal stem cells express functional respiratory complexes, which may promote in cancer cells aerobic metabolism able to counteract the progression of cancer cells that use anaerobic metabolism (Warburg effect) for their progression [179,180,181,182,183,184,185,186][61][62][63][64][65][66][67][68].
4.6. Clinical Trials on Patients Treated with SCDSFs
4.6.1. Hepatocellular Carcinoma in the Intermediate–Advanced Stage
A randomized controlled trial was conducted in 179 patients with an intermediate-advanced stage of hepatocellular carcinoma for which no treatments of consolidated efficacy were possible. On the basis of the previous studies described above, it was possible to develop a nutraceutical product containing the substances collected in the stages of cell differentiation in which they had demonstrated great efficacy in controlling tumour growth. Considering the low molecular weight of these proteins, sublingual absorption was suggested. The results showed a statistically significant difference in favour of the treated patient group, in comparison with the non-treated patient group. In all, 19.8% of the patients demonstrated a regression and 16% of the patients demonstrated a stabilization, with an overall survival after 40 months of more than 60% of the patients who responded, compared with 10% of the non-responding patients. An improvement of performance status was registered in 82.6% of the patients [187][69]. A more recent clinical trial conducted by the Scientific Institute of Research and Care Humanitas of Milan on patients with hepatocellular carcinoma in an advanced stage has confirmed the role of SCDSFs in producing a complete response [188][70].
4.6.2. Colon Cancer in an Advanced Stage
Lastly, a recent clinical trial conducted by the Institute of Oncology of University La Sapienza of Rome in a group of patients with advanced-stage colon cancer treated with Regorafenib alone, in comparison with another group treated with Regorafenib plus SCDSFs, demonstrated a statistically significant increase in survival in the latter group [189][71]. Other papers about the efficacy of SCDSFs in cancer treatments were published by different authors who suggested the use of SCDSFs as integrative treatment to the traditional therapies of consolidated efficacy [167,190,191][49][72][73]. It is also worth noting that a declaration of a committee of oncologists, published in a recent book, suggests the use of SCDSFs as integrative treatments in oncology [192][74].
References
- Duijvesz, D.; Rodriguez-Blanco, G.; Hoogland, A.M.; Verhoef, E.I.; Dekker, L.J.; Roobol, M.J.; Van Leenders, G.J.L.H.; Luider, T.M.; Jenster, G. Differential tissue expression of extracellular vesicle-derived proteins in prostate cancer. Prostate 2019, 79, 1032–1042.
- Rajagopal, C.; Harikumar, K.B. The Origin and Functions of Exosomes in Cancer. Front. Oncol. 2018, 8, 66.
- Jansen, F.H.; Krijgsveld, J.; van Rijswijk, A.; van den Bemd, G.-J.; van den Berg, M.; van Weerden, W.; Willemsen, R.; Dekker, L.J.; Luider, T.M.; Jenster, G. Exosomal Secretion of Cytoplasmic Prostate Cancer Xenograft-derived Proteins*. Mol. Cell. Proteom. 2009, 8, 1192–1205.
- Bastos, N.; Ruivo, C.F.; Da Silva, S.; Melo, S.A. Exosomes in cancer: Use them or target them? Semin. Cell Dev. Biol. 2018, 78, 13–21.
- Aghamir, S.M.K.; Heshmat, R.; Ebrahimi, M.; Khatami, F. Liquid Biopsy: The Unique Test for Chasing the Genetics of Solid Tumors. Epigenet. Insights 2020, 13, 2516865720904052.
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mech-anisms in cancer metastasis and drug resistance. Mol. Cancer. 2019, 18, 75.
- Saber, S.H.; Ali, H.E.A.; Gaballa, R.; Gaballah, M.; Ali, H.I.; Zerfaoui, M.; Elmageed, Z.Y.A. Exosomes are the Driving Force in Preparing the Soil for the Metastatic Seeds: Lessons from the Prostate Cancer. Cells 2020, 9, 564.
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51.
- Vagner, T.; Spinelli, C.; Minciacchi, V.R.; Balaj, L.; Zandian, M.; Conley, A.; Zijlstra, A.; Freeman, M.R.; Demichelis, F.; De, S.; et al. Large extracellular vesicles carry most of the tumour DNA circulating in prostate cancer patient plasma. J. Extracell. Vesicles 2018, 7, 1505403.
- Di Vizio, D.; Kim, J.; Hager, M.H.; Morello, M.; Yang, W.; LaFargue, C.J.; True, L.D.; Rubin, M.A.; Adam, R.M.; Beroukhim, R.; et al. Oncosome Formation in Prostate Cancer: Association with a Region of Frequent Chromosomal Deletion in Metastatic Disease. Cancer Res. 2009, 69, 5601–5609.
- Minciacchi, V.R.; You, S.; Spinelli, C.; Morley, S.; Zandian, M.; Aspuria, P.-J.; Cavallini, L.; Ciardiello, C.; Sobreiro, M.R.; Morello, M.; et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget 2015, 6, 11327–11341.
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420.
- Lässer, C.; Alikhani, V.S.; Ekström, K.; Eldh, M.; Paredes, P.T.; Bossios, A.; Sjöstrand, M.; Gabrielsson, S.; Lötvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 9.
- Silva, J.; Garcia, V.; Rodriguez, M.; Compte, M.; Cisneros, E.; Veguillas, P.; Garcia, J.M.; Dominguez, G.; Campos-Martin, Y.; Cuevas, J.; et al. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes Chromosomes Cancer 2011, 51, 409–418.
- Kawamura, Y.; Yamamoto, Y.; Sato, T.-A.; Ochiya, T. Extracellular vesicles as trans-genomic agents: Emerging roles in disease and evolution. Cancer Sci. 2017, 108, 824–830.
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769.
- Pan, L.; Liang, W.; Fu, M.; Huang, Z.H.; Li, X.; Zhang, W.; Zhang, P.; Qian, H.; Jiang, P.C.; Xu, W.R.; et al. Exo-somes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J. Cancer Res. Clin. Oncol. 2017, 143, 991–1004.
- Bellingham, S.A.; Shambrook, M.; Hill, A.F. Quantitative Analysis of Exosomal miRNA via qPCR and Digital PCR. In Exosomes and Microvesicles; Hill, A., Ed.; Humana Press: New York, NY, USA, 2017; Volume 1545, pp. 55–70.
- Lunavat, T.R.; Cheng, L.; Kim, D.-K.; Bhadury, J.; Jang, S.C.; Lässer, C.; Sharples, R.A.; López, M.D.; Nilsson, J.; Gho, Y.S.; et al. Small RNA deep sequencing discriminates subsets of extracellular vesicles released by melanoma cells – Evidence of unique microRNA cargos. RNA Biol. 2015, 12, 810–823.
- Mjelle, R.; Dima, S.O.; Bacalbasa, N.; Chawla, K.; Sorop, A.; Cucu, D.; Herlea, V.; Sætrom, P.; Popescu, I. Comprehensive transcriptomic analyses of tissue, serum, and serum exosomes from hepatocellular carcinoma patients. BMC Cancer 2019, 19, 1–13.
- Maia, J.; Caja, S.; Moraes, M.C.S.; Couto, N.; Costa-Silva, B. Exosome-Based Cell-Cell Communication in the Tumor Microenvironment. Front. Cell Dev. Biol. 2018, 6, 18.
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624.
- Webber, J.; Steadman, R.; Mason, M.D.; Tabi, Z.; Clayton, A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010, 70, 9621–9630.
- Zhao, H.; Yang, L.; Baddour, J.; Achreja, A.; Bernard, V.; Moss, T.; Marini, J.C.; Tudawe, T.; Seviour, E.G.; Lucas, F.A.S.; et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife 2016, 5, e10250.
- Gutkin, A.; Uziel, O.; Beery, E.; Nordenberg, J.; Pinchasi, M.; Goldvaser, H.; Henick, S.; Goldberg, M.; & Lahav, M. Tumor cells derived exosomes contain hTERT mRA and transform nonmalignant fibroblasts into telomerase positive cells. Oncotarget 2016, 7, 59173–59188.
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826.
- Rivoltini, L.; Chiodoni, C.; Squarcina, P.; Tortoreto, M.; Villa, A.; Vergani, B.; Bürdek, M.; Botti, L.; Arioli, I.; Cova, A.; et al. TNF-Related Apoptosis-Inducing Ligand (TRAIL)–Armed Exosomes Deliver Proapoptotic Signals to Tumor Site. Clin. Cancer Res. 2016, 22, 3499–3512.
- Kibria, G.; Ramos, E.K.; Wan, Y.; Gius, D.R.; Liu, H. Exosomes as a Drug Delivery System in Cancer Therapy: Potential and Challenges. Mol. Pharm. 2018, 15, 3625–3633.
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome Delivered Anticancer Drugs Across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm. Res. 2015, 32, 2003–2014.
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nat. Cell Biol. 2017, 546, 498–503.
- Wu, H.; Zhou, J.; Zeng, C.; Wu, D.; Mu, Z.; Chen, B.; Xie, Y.; Ye, Y.; Liu, J. Curcumin increases exosomal TCF21 thus suppressing ex-osome-induced lung cancer. Oncotarget 2016, 7, 87081–87090.
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92.
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818.
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285.
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170.
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014, 35, 2383–2390.
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664.
- Trivillin, V.A.; Serrano, A.; Garabalino, M.A.; Colombo, L.L.; Pozzi, E.C.; Hughes, A.M.; Curotto, P.M.; Thorp, S.I.; Farías, R.O.; González, S.J.; et al. Translational boron neutron capture therapy (BNCT) studies for the treatment of tumors in lung. Int. J. Radiat. Biol. 2019, 95, 646–654.
- Suzuki, M. Boron neutron capture therapy (BNCT): A unique role in radiotherapy with a view to entering the acceler-ator-based BNCT era. Int. J. Clin. Oncol. 2020, 25, 43–50.
- Wang, J.; Wu, W.; Jiang, X. Nanoscaled boron-containing delivery systems and therapeutic agents for cancer treatment. Nanomed. 2015, 10, 1149–1163.
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 1–15.
- Morse, M.A.; Garst, J.; Osada, T.; Khan, S.; Hobeika, A.; Clay, T.M.; Valente, N.; Shreeniwas, R.; Sutton, M.A.; Delcayre, A.; et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005, 3, 9.
- Besse, B.; Charrier, M.; Lapierre, V.; Dansin, E.; Lantz, O.; Planchard, D.; Le Chevalier, T.; Livartoski, A.; Barlesi, F.; Laplanche, A.; et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. OncoImmunology 2016, 5, e1071008.
- Muhsin-Sharafaldine, M.-R.; Saunderson, S.C.; Dunn, A.C.; Faed, J.M.; Kleffmann, T.; McLellan, A.D. Procoagulant and immunogenic properties of melanoma exosomes, microvesicles and apoptotic vesicles. Oncotarget 2016, 7, 56279–56294.
- Dai, S.; Wei, D.; Wu, Z.; Zhou, X.; Wei, X.; Huang, H.; Li, G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 2008, 16, 782–790.
- Tickner, J.A.; Urquhart, A.J.; Stephenson, S.A.; Richard, D.J.; O’Byrne, K.J. Functions and therapeutic roles of exo-somes in cancer. Front. Oncol. 2014, 4, 127.
- Gonzalez, M.J.; Seyfried, T.; Nicolson, G.L.; Barclay, B.J.; Matta, J.; Vazquez, A.; D’Agostino, D.; Olalde, J.; Duconge, J.; Hunninghake, R.; et al. Mitochondrial Correction: A New Therapeutic Paradigm for Cancer and De-generative Diseases. J. Orthomol. Med. 2018, 33.
- Bruno, S.; Collino, F.; Deregibus, M.C.; Grange, C.; Tetta, C.; Camussi, G. Microvesicles Derived from Human Bone Marrow Mesenchymal Stem Cells Inhibit Tumor Growth. Stem Cells Dev. 2013, 22, 758–771.
- Biava, P.M.; Nicolini, A.; Ferrari, P.; Carpi, A.; Sell, S. A systemic approach to cancer treatment: Tumor cell repro-gramming focused on endocrine-related cancers. Curr. Med. Chem. 2014, 21, 1072–1081.
- Fong, C.-Y.; Chak, L.-L.; Biswas, A.; Tan, J.-H.; Gauthaman, K.; Chan, W.-K.; Bongso, A. Human Wharton’s Jelly Stem Cells Have Unique Transcriptome Profiles Compared to Human Embryonic Stem Cells and Other Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2011, 7, 1–16.
- Gauthaman, K.; Yee, F.C.; Cheyyatraivendran, S.; Biswas, A.; Choolani, M.; Bongso, A. Human umbilical cord wharton’s jelly stem cell (hWJSC) extracts inhibit cancer cell growth in vitro. J. Cell. Biochem. 2012, 113, 2027–2039.
- Ayuzawa, R.; Doi, C.; Rachakatla, R.S.; Pyle, M.M.; Maurya, D.K.; Troyer, D.; Tamura, M. Naïve human umbilical cord matrix derived stem cells significantly attenuate growth of human breast cancer cells in vitro and in vivo. Cancer Lett. 2009, 280, 31–37.
- Biava, P.M.; Bonsignorio, D. Cancer and Cell Differentiation: A model to explain malignancy. J. Tumor Marker Oncol. 2002, 17, 47–54.
- Biava, P.M.; Bonsignorio, D.; Hoxa, M. Cell Proliferation Curve od Different Human Tumor Lines after in Vitro Treatment with Zebrafish embryonic extracts. J. Tumor Marker Oncol. 2001, 16, 195–202.
- Biava, P.M.; Canaider, S.; Facchin, F.; Bianconi, E.; Ljungberg, L.; Rotilio, D.; Burigana, F.; Ventura, C. Stem Cell Differentiation Stage Factors from Zebrafish Embryo: A Novel Strategy to Modulate the Fate of Normal and Pathological Human (Stem) Cells. Curr. Pharm. Biotechnol. 2015, 16, 782–792.
- Cucina, A.; Biava, P.-M.; D’Anselmi, F.; Coluccia, P.P.; Conti, F.; Di Clemente, R.; Miccheli, A.; Frati, L.; Gulino, A.; Bizzarri, M. Zebrafish embryo proteins induce apoptosis in human colon cancer cells (Caco2). Apoptosis 2006, 11, 1617–1628.
- Biava, P.M.; Carluccio, A. Activation of anti-oncogene p53 produced by embryonic extracts in vitro tumor cells. J. Tumor Marker Oncol. 1977, 12, 9–15.
- Biava, P.M.; Bonsignorio, D.; Hoxa, M.; Impagliazzo, M.; Facco, R.; Ielapi, T.; Frati, L.; Bizzarri, M. Post translational modification of the retinoblastoma protein (pRb) induced by in vitro administration of Zebrafish embryonic extracts on human kidney adenocarcinoma cell line. J. Tumor Marker Oncol. 2002, 17, 59–64.
- D’Anselmi, F.; Cucina, A.; Biava, P.M.; Proietti, S.; Coluccia, P.; Frati, L.; Bizzarri, M. Zebrafish stem cell differentiation stage factors suppress Bcl-xL release and enhance 5-Fu-mediated apoptosis in colon cancer cells. Curr. Pharm. Biotechnol. 2011, 12, 261–267.
- Proietti, S.; Cucina, A.; Pensotti, A.; Biava, P.M.; Minini, M.; Monti, N.; Catizone, A.; Ricci, G.; Leonetti, E.; Harrath, A.H.; et al. Active Fraction from Embryo Fish Extracts Induces Reversion of the Malignant Invasive Phenotype in Breast Cancer through Down-regulation of TCTP and Modulation of E-cadherin/β-catenin Pathway. Int. J. Mol. Sci. 2019, 20, 2151.
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840.
- Yu, T.; Wang, Y.; Fan, Y.; Fang, N.; Wang, T.; Xu, T.; Shu, Y. CircRNAs in cancer metabolism: A review. J. Hematol. Oncol. 2019, 12, 1–10.
- Lu, Z.; Hunter, T. Metabolic Kinases Monlighting as Protein Kinases. Trends Biochem. Sci. 2018, 43, 301–310.
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919.
- Schwartz, L.; Seyfried, T.; Alfarouk, K.O.; Moreira, J.D.V.; Fais, S. Out of Warburg effect: An effective cancer treatment targeting the tumor specific metabolism and dysregulated pH. Semin. Cancer Biol. 2017, 43, 134–138.
- Xu, X.D.; Shao, S.X.; Jiang, H.P.; Cao, Y.W.; Wang, Y.H.; Yang, X.C.; Wang, Y.L.; Wang, X.S.; Niu, H.T. Warburg Effect or Reverse Warburg Effect? A Review of Cancer Metabolism. Oncol. Res. Treat. 2015, 38, 117–122.
- Bhattacharya, B.; Omar, M.F.M.; Soong, R. The Warburg effect and drug resistance. Br. J. Pharmacol. 2016, 173, 970–979.
- Schwartz, L.; Supuran, C.T.; O. Alfarouk, K. The Warburg Effect and the Hallmarks of Cancer. Anti-Cancer Agents Med. Chem. 2017, 17, 164–170.
- Livraghi, T.; Meloni, F.; Frosi, A.; Lazzaroni, S.; Bizzarri, T.M.; Frati, L.; Biava, P.M. Treatment with stem cell differ-entiation stage factors of intermediate-advanced hepatocellular carcinoma: An open randomized clinical trial. Oncol. Res. 2005, 15, 399–408.
- Livraghi, T.; Ceriani, R.; Palmisano, A.; Pedicini, V.; Pich, M.G.; Tommasini, M.A.; Torzilli, G. Complete response in 5 out of 38 patients with advanced hepatocellular carcinoma treated with stem cell differentiation stage factors: Case reports from a single centre. Curr. Pharm. Biotechnol. 2011, 12, 254–260.
- Proietti, S.; Cucina, A.; Giuliani, A.; Verna, R.; Palombi, E.; Bava, P.M. Fish protein extract enhances clinical response to salvage chemotherapy in colon cancer patients. Org. J. Biol. Sci. 2018, 2, 81–90.
- Lugnani, F.; Simone, G.; Biava, P.; Ablin, R. The role of neuroendocrine cells in prostate cancer: A comprehensive review of current literature and subsequent rationale to broaden and integrate current treatment modalities. Curr. Med. Chem. 2014, 21, 1082–1092.
- Sell, S.; Nicolini, A.; Ferrari, P.; Biava, P.M. Cancer: A problem of developmental biology; scientific evidence for re-programming and differentiation therapy. Curr. Drug.Target. 2016, 17, 1103–1110.
- Carruba, M. Addendum: Declaration of a Committee of Oncologists. In Information Medicine, 1st ed.; Laszlo, E., Biava, P.M., Eds.; Healing Arts Press: Rochester, VT, USA, 2019; pp. 39–55.
