Photooxygenation reactions involving singlet oxygen (1O2) are utilized industrially as a mild and sustainable access to oxygenated products. Due to the usage of organic dyes as photosensitizers, these transformations can be successfully conducted using natural sunlight. Modern solar chemical reactors enable outdoor operations on the demonstration (multigram) to technical (multikilogram) scales and have subsequently been employed for the manufacturing of fine chemicals such as fragrances or biologically active compounds. This review highlights examples of solar
photooxygenations for the manufacturing of industrially relevant target compounds and discusses current challenges and opportunities of this sustainable methodology.
- solar chemistry
- photooxygenations
- solar reactor
- chemical manufacturing
1. Introduction
Photochemistry has recently seen a remarkable renaissance in synthetic organic chemistry [1][2][3][4][1,2,3,4]. This trend was sparked by the development of new photochemical transformations [5][6][7][8][5,6,7,8] as well as advances in photoreactor and light technologies [9][10][11][12][13][9,10,11,12,13]. However, the large-scale realization of photochemical manufacturing processes is hindered by their quantum yields and the significant installation and maintenance costs of artificial light sources [14][15][14,15]. As a result, industrial photochemistry is restricted to low-volume but high-value fine chemicals (such as fragrances, flavors or vitamins), or bulk but low-value chemicals (such as haloalkanes, oximes, sulfonyl chlorides and sulfonic acids) [14][15][16][17][18][19][14,15,16,17,18,19]. Natural sunlight is regarded as an alternative and sustainable energy source for a range of photochemical transformations [20][21][22][23][20,21,22,23]. Up until the early 20th century, photochemical experiments were routinely performed by placing sealed flasks and tubes in direct sunlight [24][25][26][27][24,25,26,27]. Although this ‘flask in the sun’ approach is still followed today [28][29][28,29], the small scales and prolonged exposure times result in very low productivities of the desired products. Recently, sunlight collectors and concentrators that were initially developed for energy generation [30][31][32][33][30,31,32,33] have been modified for solar photochemical experimentation [34]. Technical-scale solar degradation and detoxification processes have subsequently been successfully realized using these technologies [35][36][37][38][35,36,37,38]. In contrast, however, preparative solar chemistry for the manufacturing of chemicals is still comparably rare [39][40][41][42][43][44][45][46][47][39,40,41,42,43,44,45,46,47]. The main hurdles for a widespread implementation of solar synthesis are the discontinuous availability and the low UV content (<400 nm) of just 3–5% of natural sunlight [48]. Sunlight also consists largely of direct and diffuse radiation and their ratios vary with the location and weather [49]. Solar manufacturing may thus be ideally performed in the visible range (400–700 nm: 42–43% of solar spectrum [48]) for small annual production targets (<100 tons/year) that do not require continuous, year-round operation under optimal solar light conditions. Photooxygenations with singlet oxygen (1O2) combine catalytic amounts of a dye sensitizer with visible light and yield a variety of oxygenated products [50][51][52][50,51,52]. Photooxygenations are also performed industrially to produce low-volume fine chemicals such as fragrances, flavors or pharmaceuticals [53][54][55][53,54,55]. These features make photooxygenations ideal candidates for solar chemical manufacturing processes.
2. Solar Reactors for Synthetic Applications
Solar reactors are classified based on their sun concentration factor (CF) and range from non- to highly concentrating systems. Non- and low concentrating reactors are characterized by large apertures that enable them to harvest direct and diffuse radiation. The simplest non-concentrating devices are flatbed reactors with large surface areas, thin bodies, and operation volumes of up to 30 L (Figure 1a) [56]. These fixed reactors are best tilted to the latitude of the location for optimal harvest of sunlight. Improved models operate in circulation mode with an external heat exchanger. The compound parabolic collector (CPC) uses a ‘round W’-shaped polished aluminum reflector that is tilted to the local latitude and directs all available radiation onto a central received tube (Figure 1b) [57][58][57,58]. These advanced systems have a concentration factor of one sun (or slightly above) and most commonly operate in circulating batch mode on volumes of up to 100 L. Several reactors can also be connected in series to form larger units. Parabolic trough reactors utilize large reflectors that focus only direct sunlight onto a reaction tube in their focal line and can reach concentration factors of 20–150 suns [32]. More modern reactors such as the former PROPHIS loop (PaRabolic trough-facility for Organic PHotochemical synthesis, Figure 1c) operate on volumes up to 150 L and track the movement of the sun, either horizontally or three-dimensionally (horizontally and vertically) [59].

Figure 1. (a) Circulating flatbed reactor with reflective back and external cooling at James Cook University in Townsville, Australia. (b) CPC reactor with a 2 m2 aperture at James Cook University in Townsville, Australia. (c) Former PROPHIS loop at the German Aerospace Center (DLR) in Cologne-Porz, Germany (modified from [60]).
While solar dish concentrators can provide very high concentration factors of up to 5000 suns, these sophisticated devices are rarely used for preparative solar photochemical applications. Since the receiver in the focal point easily reaches extreme temperature ranges, dish reactors are instead advantageous for thermochemical processes [33]. Likewise, solar furnaces can reach extreme concentration factors of 5000–20,000 suns by reflecting sunlight via a heliostat or heliostat field onto a concentrator, which then focuses the sun’s beam onto the experimental setup. The naturally extreme heat generation and the costly installation have limited the application of solar furnaces mainly to material sciences and solar thermal research activities [61][62][63][61,62,63].
3. Photooxygenations in Organic Synthesis
Photooxygenations, also known as Type II photosensitized oxidations [64], utilize singlet oxygen (1O2), which is generated from ground-state triplet oxygen (3O2) via photosensitization (Scheme 1) [65]. The sensitizer (Sens) initially absorbs light and undergoes intersystem crossing (ISC) to its triplet excited state (3Sens*). Subsequent energy transfer to ground state oxygen produces singlet oxygen. The dissolved oxygen concentration in most organic solvents is low [66][67][66,67] and thus, fine streams of oxygen or compressed air are constantly passed through the reaction media. Likewise, the lifetime of singlet oxygen critically depends on the reaction medium and is highest in halogenated solvents [67]. Due to the environmental hazards linked to these solvents, many industrial and solar photooxygenation processes are conducted in less harmful alcoholic solvents instead [68]. A series of photosensitizers with large absorption coefficients within the visible spectrum, suitable solubilities in a range of organic solvents, long triplet lifetimes and high quantum yields for 1O2 generation have now become available [69][70][69,70]. The most common photosensitizers methylene blue, tetraphenylporphyrin and rose bengal (as disodium salt) are shown in Scheme 1. For easy recovery and reuse, photosensitizers have also been immobilized on an inert solid support [71][72][71,72]. Photooxygenations have furthermore been successfully performed under continuous-flow conditions [73]. Thermal oxygenation processes involving 1O2 have likewise been developed but are at present less important in organic synthesis [74].
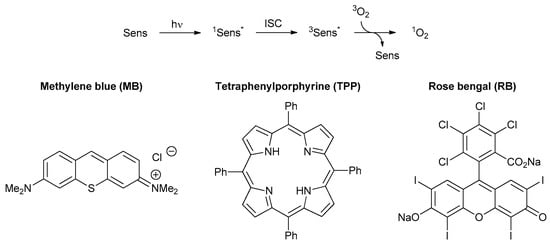
Scheme 1.
Photosensitized generation of singlet oxygen and common photosensitizers.
Singlet oxygen can react with a variety of functional groups (Scheme 2) [50][51][52][50,51,52]. Conjugated dienes preferably undergo [4+2] cycloadditions to endoperoxides, while electron-rich olefins favor [2+2] cycloadditions to 1,2-dioxetanes. Inactivated olefins with allylic hydrogen atoms undergo Schenck-ene-type reactions to allylic hydroperoxides instead [75][76][75,76]. Thioethers and phosphines produce the corresponding sulfoxides and phosphine oxides. All reaction types incorporate molecular oxygen into a molecular entity with high to quantitative atom economies.
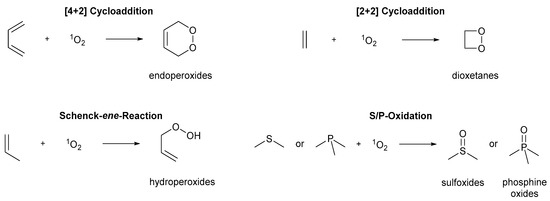
Scheme 2. General photooxygenation reactions.
Many of the industrially relevant photochemical processes convert naturally occurring or semi-synthetic starting materials to their corresponding oxygenated products [53][54][55][53,54,55]. Of these, essential oil derived starting materials are especially common [55][77][55,77], which makes photooxygenations promising applications for product diversification and value-adding in the essential oil industry [78]. This review will focus on demonstration (multigram)- to technical (multikilogram)-scale transformations conducted in purpose-designed solar reactors. Whenever available, key technical features of the solar reactor systems used have been provided. Related demonstration-scale photooxidations involving the super oxide anion radical (O2•–) or a thermal oxidation step were also included. In many cases, solar transformations were monitored, and obtained product mixtures analyzed by GC or GC–MS analysis techniques. Whenever necessary, the reported yields or conversions from these studies were rounded for consistency and to account for experimental errors.
