Ovarian cancer is the eighth-most common cause of death among women worldwide. In the absence of distinctive symptoms in the early stages, the majority of women are diagnosed in advanced stages of the disease. Surgical debulking and systemic adjuvant chemotherapy remain the mainstays of treatment, with the development of chemoresistance in up to 75% of patients with subsequent poor treatment response and reduced survival. Therefore, there is a critical need to revisit existing, and identify potential biomarkers that could lead to the development of novel and more effective predictors for ovarian cancer diagnosis and prognosis. The capacity of these biomarkers to predict the existence, stages, and associated therapeutic efficacy of ovarian cancer would enable improvements in the early diagnosis and survival of ovarian cancer patients.
- ovarian cancer
- biomarkers
- tumour mutation burden
- DNA repair pathways
- cell-cycle-related genes
1. Introduction
Figure 1. These biomarkers are already known to be overexpressed or deregulated in various signalling pathways in women with ovarian cancer [1]. Evidently, ovarian cancer is not a common disorder but includes a heterogeneous community of tumours that affect various molecular genetic mechanisms and putative molecular signalling pathways [2]. The main signalling pathways targeted for ovarian cancer diagnosis, progression and treatment are outlined in the following sections.
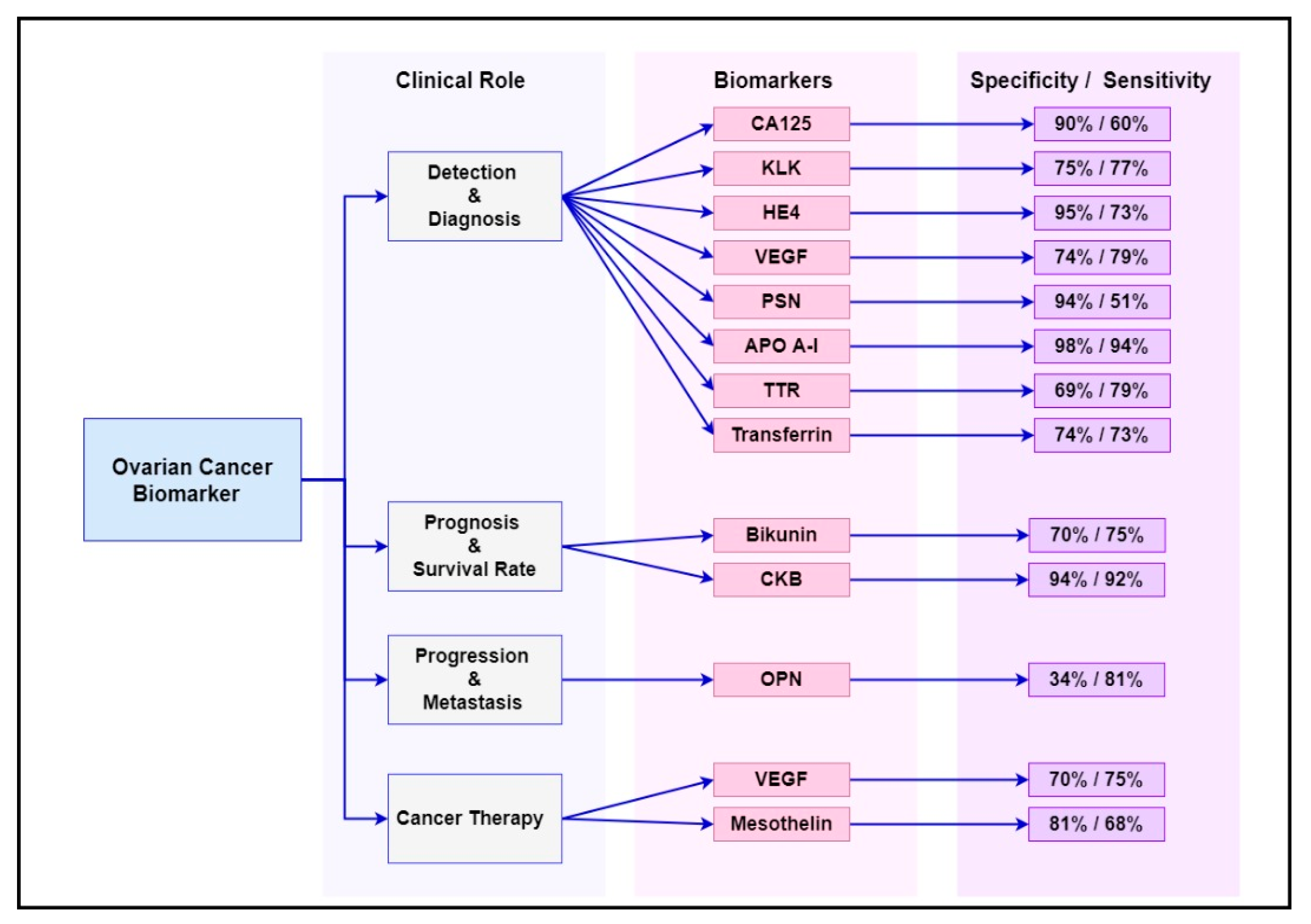
Figure 1.
2. Current Biomarkers Associated with Diagnosis, Progression and Treatment Response of Ovarian Cancer
2.1. Carbohydrate Antigen 125 (CA125)
Cancer antigen 125 or carcinoma antigen 125, also known as MUC16, is a protein encoded by the MUC16 gene [3]. Clinically, it is used as a diagnostic test to measure the amount of the protein CA125 in the serum. In most laboratories, the normal value for CA125 is 0 to 35 units/mL.
Up to 80% of women diagnosed with late-stage epithelial ovarian cancer have elevated CA125 levels in their serum [4]. Unfortunately, CA125 has limited usefulness in detecting ovarian cancer in the early stages, as only 50% of these cases had elevated CA125 levels [5]. In addition, many other conditions can also cause the elevation of CA125 levels, including endometriosis, liver cirrhosis, normal menstruation, pelvic inflammatory disease and uterine fibroid. Therefore, this antigen lacks the specificity and sensitivity to be considered a reliable biomarker for the early detection of ovarian cancer [6]. CA125 is found to be more sensitive and specific in postmenopausal women than in premenopausal women. Serum CA125 is incorporated into the Risk Malignancy Index (RMI) algorithm. The RMI is widely used as a risk assessment of ovarian malignancy in clinical practice. The RMI score is generated by the simplified serum CA125 level regression equation, the menopausal status score and the ultrasound features score (RMI = ultrasound findings × menopause status × CA125 U/mL). The use of the RMI has a higher sensitivity of 87% and a specificity of 97% for ovarian cancer detection compared to CA125 alone [7].
In a recent study using a training and confirmation cohort, four existing clinical tests available for the diagnosis of ovarian cancer (RMI score and ROMA, CA125 and HE4) and a panel of 28 immunosoluble biomarkers from 66 patients undergoing surgery for suspected ovarian cancer were assessed through a multiplex immunoassay. Using a two-step triage model for women with presumed ovarian mass, IL-6 > 3.75 pg/mL was established as the main triage, supplemented by standard testing (CA125 or RMI score) for ovarian cancer in patients greater than CA125 or RMI alone (misclassification rate 4.54–3.03 percent vs. 9.09–10.60). Therefore, in conjunction with traditional studies, IL-6 can be a beneficial therapeutic biomarker for the triage of patients with potential malignant ovarian mass [8]. The reproducibility of IL6 measurement may be a challenge in clinical practice as IL6 level can increase if infections and or inflammatory conditions occur.
2.2. Osteopontin (OPN)
Osteopontin is an adhesive glycophosphoprotein secreted by activated T lymphocytes, macrophages, and leukocytes, and found in the extracellular matrix, sites of inflammation and body fluids [9] Osteopontin is not only expressed in ovarian cancer but also in endometrial, cervical, breast, colorectal, nonsmall cell lung, prostate, hepatocellular and gastric cancer. OPN is associated with tumour progression, invasion and metastasis. In 2001, OPN was identified with a cDNA microarray system using RNA isolated from several ovarian cancer cell lines, with surface epithelial cells as controls [10][11]. The levels of OPN were also significantly higher (
p
n
n
n
n = 47) [12].
In addition, OPN has been utilised to predict the progression of disease in advanced EOC, as the prognosis of patients with peritoneal spread is poor. The levels of osteopontin in 32 out of 40 peritoneal metastatic biopsies were found to be significantly elevated compared to the levels found in primary ovarian tumour tissues among women with Stage III EOC [13]. In addition, the elevated OPN levels were independently correlated with extremely poor prognosis among these women (
n
n = 8). Furthermore, the high levels of osteopontin could be measured in the urine samples of patients with high-grade ovarian cancer, so this test could potentially be used clinically as a noninvasive tool for the early diagnosis of ovarian cancer [14].
2.3. Kallikreins (KLKs)
Kallikreins are a subgroup of serine proteases with different physiological roles. The human kallikrein gene family has now been entirely defined to include 15 members on chromosome 19q. They are expressed in epithelial and endocrine tissues regulated by hormones in cancer and they are shed and detected in human body fluids [15]. Therefore, many studies have been carried out to find their role in cancer diagnosis and prognosis. A total of 12 out 15 KLKs are upregulated in ovarian cancer, with some KLKs correlating to poor prognosis and late-stage disease (4–7, 10 and 15), as well as chemoresistance (KLK 4 and 7) to a first line paclitaxel agent [16].
In a study by Luo et al., the preoperative serum level of human kallikrein (hk10) in 146 patients with ovarian cancer was significantly elevated compared to 97 healthy women and 141 women with benign gynaecological diseases [17].
2.4. Bikunin
Bikunin is a multifunctional glycoprotein, which mediates the suppression of tumour cell invasion and metastasis. The measurement of bikunin levels in the tissue of patients with malignant diseases has been introduced as a simple diagnostic tool for the evaluation of the prognosis. High preoperative bikunin levels have been reported to be a strong favourable prognostic marker for ovarian cancer [18]. Matsuzaki et al. found, in an extensive study, that bikunin protein in the plasma of women with ovarian cancer (
N
N
N
p
p = 0.023) [18]. Measuring levels of bikunin in plasma is easy and relatively inexpensive; therefore, it has the potential to be included as a prognostic biomarker for ovarian cancer. However, there is a significant overlap in bikunin levels across cancer, benign and healthy controls, which needs to be further investigated before it can be of clinical use.
2.5 Human Epididymis Protein 4 (HE4)
Also known as WAP 4-disulphide core domain 2 (WFDC2), HE4 was first introduced as an ovarian cancer biomarker in 1999 [19]. The expression of HE4 is associated with cancer cell adhesion, migration and tumour growth, which can be related to its effects on the EGFR-MAPK signalling pathway [20]. Many studies suggested that HE4 is absent in normal ovarian surface epithelium but is expressed specifically in 100% of human endometrioid epithelial ovarian cancers (
n
n = 60) [21]. An ELISA analysis of serum HE4 levels in 37 patients with ovarian cancer, compared with 65 healthy controls, showed that HE4 had the same specificity and sensitivity as CA125 and detected fewer false positives in patients without a malignant disease [7].
The marker HE4 is significantly increased in ovarian and endometrial cancer, but not in endometriosis. HE4 can be increased, although it is less frequently elevated than CA125 in patients with benign disease, especially in premenopausal patients. The alternate probability of a malignancy algorithm (ROMA) blends the values of CA125 and HE4 with menopausal status in the predictive index and has been shown to stratify patients into high and low risk categories, with differing outcomes across many trials [22].
2.6. Vascular Endothelial Growth Factor (VEGF)
VEGF is a vascular permeability factor that is a key regulator of physiological and pathological angiogenesis, and makes a major contribution to tumorigenesis [23]. VEGF levels are known to be elevated in patients with ovarian cancer and contribute to the accumulation of ascites [24]. An analysis associated with VEGF levels in the preoperative sera of 314 patients with ovarian cancer recorded that higher VEGF levels were separately correlated with shorter survival periods [25]. In addition, tumour samples from 18 patients with advanced stage serous epithelial ovarian cancer were evaluated for VEGF expression by a reverse-transcriptase polymerase chain reaction (RT-PCR) [26]. It was demonstrated that 12 samples were found to be strongly positive, whereas six samples had low/negative VEGF expression. The median survival was longer, at 60 months in the VEGF-low/negative group compared to 28 months in the VEGF-positive group (
p
BRCA-mutated disease [27].
2.7. Human Prostasin (PSN)
PSN is a trypsin-like proteinase (40 KDa) found on chromosome 16p11.2. It plays a major role in the activation of epithelial sodium channels and in the reduction of invasive prostate and breast cancers in vitro [28]. Similarly, the epidermal tight junction forming and terminal differentiation are related to the matriptase-prostasin proteolytic pathway [29].
The potential use of prostasin as a novel biomarker for ovarian carcinoma was proposed by Mok et al. using microarray technologies to classify upregulated genes for secretive proteins [30]. The findings revealed an overexpression of PSN in malignant epithelial ovarian cells and stroma, relative to standard ovary tissue, with a sensitivity and specificity of 51.4% and 94%, respectively [31]. Gene expression analysis indicated that PSN was expressed in ovarian cancer at levels more than 100 times greater than those found in normal or benign ovarian lesions. This overexpression signature was found in the early stages of ovarian cancer and maintained in the higher stages and grades [32]. Costa et al., on the other hand, reported a slightly higher overexpression of mRNA prostasin in freshly frozen ovarian cancer tissues than in usual controls. Thus, it has the ability to be used clinically as a differential diagnostic marker for ovarian cancer [32]. In another study by Mok et al., the combination of CA125 and prostasin gave a sensitivity of 92% and a specificity of 94% for detecting ovarian cancer [30].
2.8. Creatine Kinase B (CKB)
Creatine kinase plays a crucial function in the energy homeostasis of vertebral cells. CKB is a cytosolic isoform of creatine kinase that displays upregulated expression in a number of cancers. It has been reported that certain ovarian cancer tissues have improved protein CKB expression [33]. In addition, CKB decreased the intake of glucose and lactate, and improved the ROS output and consumption of oxygen. As a result, it was indicated that the suppression of CKB induced G2 arrest in the cell cycle through the PI3K/AKT and AMPK pathways. Clinically, this mechanism has helped clinicians to use this biomarker in cancer cell survival and tumour progression. CKB activity measured in preoperative serum samples was higher in women with ovarian cancer (
N
N
N
p = 0.0096 [34]. CKB is highly expressed in early stage ovarian tumour tissues and is, therefore, a potential biomarker for the early detection of ovarian cancer; it should be further investigated [34].
2.9. Mesothelin
Mesothelin, a tumour differentiation antigen found in mesothelial pleura, peritoneum and pericardium, was discovered in 1996 at the National Cancer Institute [35]. Mesothelin is widely expressed in many tumours, including 70% of ovarian cancers. Several mesothelin-directed treatments have been studied in clinical trials, including antimesothelin immunotoxins and antibody-drug conjugates (ADC) [36]. Quanz et al. showed the activity of anetumabravtansine in conjunction with conventional chemotherapy in ovarian cancer models. Anetumabravtansine is an ADC that produces a human antimesothelin antibody conjugated by a reducible disulphide linker to the DM4maytansinoid tubulin inhibitor. Both in vitro and in vivo experiments have indicated the selective activity of anetumabravtansine in injecting new expression cells and tumours, including low-sensitivity (68.2%) and high-specificity (80.5%) ovarian cancer [37]. In animal models with ovarian cancer, treatment with anetumabravtansine exhibits improved potency in combination with carboplatin, compared to either drug alone [37]. Similarly, Anetumabravtansine also demonstrates enhanced antitumour efficacy when combined with Bevacizumab, an anti-VEGF agent. A phase 1b study (NCT02751918) using anetumabravtansine in combination with pegylated liposomal doxorubicin in ovarian cancer patients is ongoing.
2.10. Apolipoprotein A-I (apoA-I)
ApoA-I is a high-density lipoprotein (HDL) and apolipoprotein A-I in plasma. Apo A-I levels have been reported to decrease in the sera of patients with ovarian cancer [38]. A multiplexed magnetic nanoparticle-antibody conjugates (MNPs-Abs) based fluorescence spectroscopic system analysis combining CA125, β2-M and ApoA1 for the early detection of ovarian cancer performed by Pal et al. found that while CA125 detection only identifies 50–60% of early stage ovarian cancer, the combination of the three biomarkers achieved high sensitivity (94%) and high specificity (98%) in distinguishing early stage ovarian cancer patients from healthy individuals [39]. This proposed multiplexed panel assay is also cost-effective, and further clinical investigation should be conducted to develop a clinically beneficial test kit.
2.11. Transthyretin (TTR)
TTR is a natural serum protein synthesised mostly in the liver [40]. It attaches and transports the thyroid hormones and retinol protein binding to the retinal complex [41]. Low TTR serum levels were found in ovarian cancer and used with other biomarkers to detect ovarian cancer [42][43]. Using liquid chromatography with tandem mass spectrometry, Kozak et al. found that TTR, in combination with beta-haemoglobin, apolipoprotein AI, transferrin and CA125, significantly improved the detection of early stage ovarian cancer [43]. TTR was found to be an important marker for the detection of stage I–II ovarian cancer, with a sensitivity and specificity of 78.6% and 68.8%, respectively.
2.12. Transferrin
Transferrin is essentially synthesised in hepatocytes and responsible for delivering plasma iron to the cell. It plays a major role in cell division and proliferation [6]. Ahmed et al. documented the downregulation of transferrin in the sera of patients with ovarian cancer [44]. In another case-control study, the level of transferrin was measured using an immunological turbidimetric assay in the sera of 37 women with ovarian cancer and compared to those with benign ovarian diseases (
N
N = 31). It was found that the use of the biomarker transferrin as a detection tool for ovarian cancer has only low sensitivity and specificity, at 72.9% and 74.1%, respectively [45]. Therefore, transferrin needs to be used in combination with other biomarkers to achieve clinical significance.
3. Emerging Biomarkers Associated with Ovarian Cancer Diagnosis and Prognosis
The diagnosis of ovarian cancer is currently focused on restricted imaging techniques and the concentration of certain biomarkers circulating with established levels of sensitivity and specificity. New biomarkers are required to complement and improve the efficacy of the existing clinical tests. New biomarkers, including circulating DNA tumours, serum tumour proteins, circulating cancer cells or serum metals such as Cu and zinc, are emerging to complement the clinically available diagnostic methods [46][47]. A summary of these new biomarkers is shown in
3.1. Cu Isotope
The concentration of Copper (Cu) in the bloodstream is regulated by two major organs: it is absorbed by the intestine and transported to the liver [48]. Changes in the concentration of Cu influenced by modified metabolic processes can affect health and disease [49]. In recent study looking at copper composition
65
63
65Cu), in blood samples from 44 ovarian cancer patients, and 13 ovarian biopsies using multicollector inductively coupled plasma mass spectrometry, a connection has been demonstrated to cancer progression [50]. The copper isotope ratio δ
65
n
n
63
3.2. Exosomes
Exosomes are endocytic and heterogeneous membrane-derived vesicles that are actively secreted by various forms of cell, and they can be visualised by electron microscopy [51]. Recent studies have reported the role of exosomes in immune regulation [52], intercellular communication [53][54] and biological events such as the coagulation [55] and microenvironmental regulation of tissues [56], as well as their role in the development of cancer, metastases and drug resistance [57][58].
In addition, a recent clinical trial found that the levels of exosomes were three to four times higher in the circulation of ovarian cancer patients compared to normal individuals [59]. As a result, a growing interest in determining the therapeutic importance of these nanoparticles in cancers has contributed to the discovery of either tissue or disease-specific exosome material, such as nucleic acids, proteins and lipids, as a source of new biomarkers [60]. While exosomes are known to be ideal biomarkers in the diagnosis of cancer due to their unique characteristics, there is still a long way to go in developing exosome-based assays.
3.3. lncRNA and mRNA Biomarkers
The first comprehensive study, conducted in 2019, investigated the lncRNA–miRNA–mRNA networks and lncRNA–RNA binding protein-mRNA networks in ovarian cancer, and confirmed the presence of some lncRNAs and mRNAs in ovarian cancer cell models [61].
In EOC, some lncRNAs were found to be differentially expressed compared with benign and normal tissues, which demonstrated the up- or downregulation of 663 lncRNAs [64]. Based on a systematic analysis of the profiles of lncRNA and mRNA expression from the Cancer Genome Atlas (TCGA), a platinum resistance-specific lncRNA-mRNA network was discovered involving a total of 124 significant lncRNA-mRNA coexpression relationships that primarily regulate metabolic pathways, indicating the prognostic and therapeutic potential of lncRNAs in high-grade serous ovarian cancer [65].
3.4. Aldehyde Dehydrogenase 1 (ALDH1)
A member of the aldehyde dehydrogenase protein family, ALDH1A1 expressed in a subpopulation of tumour-initiating cells and is therefore a potential candidate biomarker for cancer therapy. In a recent study by Chang et al. utilising an immunohistochemistry staining microarray study, the possible functions of ALDH1 in ovarian cancer consisted of the diagnosis of tumour type and disease staging, as well as therapeutic responses and overall survival rate. The data have shown that ALDH1 expression has been linked with longer average patient survival and that elevated ALDH1 expression is a positive prognosis factor in patients with ovarian cancer [66].
+ stem cell clones [67]. Hence, ALDH1 may be a useful biomarker for the identification of tumorigenic stem cells. Similarly, high ALDH1 expression in tumour cells was significantly associated with histological subtypes, the early FIGO stage, a well-differentiated grade and better survival probability (
p
p > 0.05) [68].
3.5. Folate Receptor Alpha (FOLR1)
FOLR1 is a membrane-bound receptor protein that is active in the movement of folate to cells and other cellular processes. The overexpression of FOLR1 was found in 69% of uterine serous carcinoma [69] and rapidly dividing cells. The expression of FOLR1 is regulated by the loss of extracellular folate levels, the accumulation of homocysteine, steroid hormone levels and genetic mutations. An initial study suggested a significant correlation between folate levels and tumour aetiology, and folate levels and progression, with suggestions for future research in FOLR1 gene expression and regulation [70].
In addition, the overexpression of FOLR1 has been documented in various epithelial nonmucinous tumours, including ovarian carcinoma; however, its assessment as a novel biomarker for early detection has not yet been verified. In either case, the overexpression of FOLR1 was reported in serous ovarian carcinoma describing clinicopathological characteristics and outcomes, as well as the relationship between FOLR1 and chemoresistance [10].
3.6. Glutathione S-Transferase Polymorphisms
S-transferase family (GSTM1, GSTT1, and GSTP1) are the result of major structural gene deletions, which in turn control the metabolism of drugs and impair chemotherapy in cancer patients. The GST polymorphisms are highly expressed in human ovaries [71]. Earlier epidemiologic studies did not confirm the association of GST polymorphisms with epithelial ovarian cancer [72], while it was proposed that in persons with homozygous deletions of GSTM, where GSTT had decreased or where there was no GST involvement, the removal of electrophilic carcinogens was challenging.
In addition, a recent analysis utilising DNA extracts from epithelial ovarian cancer tissue in which the GSTT1, GSTM1 and GSTP1 genotypes were identified using multiplex PCR and PCR-RFLP indicated that the combination of no GSTM1 and low GSTP1 resulted in over 60% improvement in progression-free survival and almost 40% improvement in overall survival [73]. Similarly, a meta-analysis investigating the relationship of GST polymorphisms with ovarian cancer risk suggested that the role of GSTs is highly significant in drug-resistant tumours where the higher expression of GSTs could alter the control of the kinase cascade during drug therapy [74].
