Zinc is a micronutrient involved in many fundamental roles that are vital for routine bodily functions. A tight control of its concentration, however, is necessary to ensure balance inside the cells. Zinc transporters are thus essential to maintain cellular homeostasis.
- zinc
- TMEM163
- Cation diffusion facilitator
- ZnT
1. Introduction
Zinc is a micronutrient involved in many fundamental roles that are vital for routine bodily functions. It is estimated that one in ten proteins have zinc-binding motif [1]. Additionally, every one of the main six classes of enzymes (hydrolases, isomerases, ligases, lyases, oxidoreductases, and transferases) contains zinc-dependent proteins [2]. At the molecular level, zinc binds to proteins [1][3] in various capacities such as protein structural integrity, catalysis, and regulatory activity of DNA function [2][4]. Additionally, zinc plays other crucial roles in cell signaling pathways such as the “zinc spark” during fertilization [5], modulation of neurotransmitter receptors or ion channels [6][7][8] and second messenger system of specific signal transduction pathways [1]. The widespread effects of and dependence on zinc by various proteins demonstrate the broad usefulness of this trace metal. We refer the reader to a review on the multi-dimensional effects of zinc in cells by Cuajungco, Ramirez, and Tolmasky (2021) as part of the Special Issue.
Mammals, especially in humans, require zinc for proper immune responses. Innate and adaptive immune systems both rely on proper zinc levels in order to develop and carry out their protective duties [2][3]. Evidence also demonstrates that zinc can benefit those suffering from viral, bacterial, and even parasitic infections [2]. In addition to helping to destroy endocytosed pathogens, zinc can be used in ridding cells of reactive oxygen species (ROS) [2]. However, for some cell types, there is indication demonstrating that intracellular zinc accumulation leads to increased ROS formation in the mitochondria [9][10]. The strict control of ROS production demonstrates another crucial, although indirect role for zinc, especially that ROS imbalance releases zinc from metalloproteins and creates oxidative stress within the cells leading to disease [11][12].
Zinc dyshomeostasis is a known factor in many human health problems. In diabetes mellitus (DM), there is evidence associating zinc imbalance occurring at the physiological and cellular levels [11][13]. For example, insulin needs two zinc ions to stabilize its granules, but the role of zinc in DM is more complicated than simply zinc dyshomeostasis or abnormal metabolism [14], which will be discussed later in this review. Zinc deficiency also has consequences on the immune system. One such example is that zinc deficiency adversely affects the function of immune cells as evidenced by atypical inflammatory responses occurring under reduced zinc conditions [15]. Additionally, zinc deficiency has been shown to be associated with chronic diseases such as liver cirrhosis [16] and asthma [3]. While cases of zinc deficiency in human population are often more mild-to-moderate in their severity, it is relatively widespread and affects one in four people [3][11]. With zinc deficiency afflicting many people worldwide and a wide variety of health issues stemming from it, nutritional supplementation appears to be quite important. Nevertheless, zinc supplementation alone is not enough to solve the many issues in human health [3], because abnormal zinc levels in the human body could also mean increased tissue or cellular zinc concentrations that result in cytotoxicity. Thus, it is necessary to further understand zinc homeostasis with respect to the living cell.
2. Zinc Transport
Further insight into human diseases linked with zinc imbalances can be obtained by studying how zinc is transported into and out of cells. The solute carrier 30 (SLC30) and the SLC39 families comprise the two major groups of cellular zinc transporters [17]. As such, these families play vital roles in regulating tissue zinc homeostasis. Due to their considerable control of zinc concentrations, it is not surprising that a large number of diseases can result from, or are associated with, members of these two families that are either dysfunctional or differentially expressed [18][19]. Due to the similarities of human Transmembrane 163 protein (TMEM163) to SLC30 proteins, as will be discussed later on, only the SLC30 family will be further explored in this section.
2.1. The SLC30 Family or ZnT Efflux Transporters
The SLC30 proteins or ZnTs belong to the CDF superfamily [18][20]. These are zinc transporters found across a wide variety of species from bacteria to humans. ZnTs have six transmembrane domains (TMDs) with intracellular amino terminus domain (NTD) and relatively longer carboxyl terminus domain (CTD) [18][21]. Although a longer CTD is expected of CDF proteins, there have been protein homologs identified in bacteria (marine and soil) that lack longer CTD [22]. There are currently ten known ZnT proteins [18][20] with ZnT1–ZnT4 identified through direct experimentation with zinc-resistant cells or via cloning, while ZnT5–ZnT10 were found by homology sequence analysis using previously discovered ZnTs [18]. The structure of a bacterial CDF called YiiP has been published [23], which served as a template to model theoretical structures of certain mammalian ZnT proteins. Recently, however, the structure of human ZnT8 was also solved using cryo-electron microscopy [24]. The recent publication of the ZnT8 structure confirmed that certain members of the CDF family, especially the SLC30 proteins, have specific zinc-binding sites within their TMDs and the CTD [24].
As a whole, the ZnTs function almost exclusively as zinc effluxers, and as such, they export zinc from the cytosol to either the outside of the cell or bring cytoplasmic zinc into vesicles or organelles [18][20]. Note, however, that ZnT10 is unusual from its related members in that it has been reported to efflux not only zinc [25], but also manganese ions [17][26][27]. Recent data suggest that ZnT10 mainly extrudes manganese [27] and that manganese efflux appears to be a calcium-dependent antiport process [28] as opposed to a proton-dependent mechanism previously shown for certain ZnT proteins [29][30][31]. The functional difference between ZnT10 and other ZnTs may stem from the putative metal binding motif on ZnT10′s TMD2, which is NXXXD compared to the typical HXXXD associated with zinc binding observed for specific ZnTs [17]. Counter to expectation, site-directed mutagenesis (SDM) targeting both asparagine (N) and aspartate (D) of the TMD2 NXXXD motif within ZnT10 did not inactivate its manganese efflux function [26][27]. These results indicate that the TMD2 HXXXD motif that has been ascribed to zinc binding is not generalizable and cannot be used solely to classify a protein as a ZnT family member without sufficient empirical evidence to support classification. A case in point, ZnT6 does not have the TMD2 HXXXD motif, but rather, it has a TMD2 DXXXD motif. As we will elaborate later on, the TMD2 DXXXD motif is also found in both YiiP and TMEM163 [32]. Despite such debatable findings, the role of ZnT10 as an effluxer is not in doubt; however, it may be that this protein is an atypical ZnT member as is the case for ZnT9 and TMEM163 as discussed below and in the subsequent section. Future research should investigate specific motifs within TMD2 and TMD5, as well as relevant amino acid residues surrounding these domains to fully define their role in zinc transport and help establish a way to define classification and membership to the ZnT family or the CDF family, in general.
A truncated isoform of ZnT5 [33] has been reported to act as a zinc influxer, while a variant of ZnT8 [34] implicated in DM appears to show a similar influx activity when heterologously expressed in cells. While such bidirectional activity may suggest a novel function for some of these members of the ZnT family, this characteristic has been called into question upon further examination of the structure of ZnT8 [24][35]. Thus, members of the SLC30 family should be recognized as mainly effluxers until more extensive evidence showing bidirectional function is experimentally validated. Of added interest is that ZnT9 had its membership with the SLC30 family called into question due to its reported role as a nuclear receptor co-activator and possible lack of zinc transport function [17]. Recently, however, research into the causal role of a point mutation within ZnT9 leading to a cerebral-renal disease in humans has shed some light in support of ZnT9 as a zinc transporter [36]. Moreover, knock-down of ZnT9 expression in human chondrocytes results in upregulation of the zinc-regulatory transcription factor, MTF-1, while over-expression of ZnT9 produces an opposite effect, suggesting that ZnT9 mediates intracellular zinc flux [37]. Noteworthy is that ZnT9 also has the HXXXD zinc-binding motif on TMD2 that is found among members of the ZnT family [17]. To resolve this controversy, further studies would need to be done to validate these observations and to show specific domains or motifs that are directly responsible for the zinc efflux function of ZnT9.
ZnTs localize to different areas in the cell. Some common subcellular localization of ZnTs include the plasma membrane, synaptic or secretory vesicles, and the Golgi apparatus [17][18][20]. A central structural feature of ZnTs is that they transport zinc as a dimer. Noteworthy is that certain ZnT subunits heterodimerize with each other to carry out their functions, although some ZnT subunits can only form functional homodimers while others could form more than one heterodimer version [18][38][39]. Strong evidence shows that ZnT1–ZnT4, ZnT7 [39], and ZnT8 [40] form homodimers, but that ZnT1–ZnT4 are also able to form heterodimers with each other [38]. Similarly, ZnT5 may form a homodimer but it also heterodimerizes with ZnT6 [39][41]. ZnT3 and ZnT10 are reported to form heterodimers with each other [42]. As heterodimers, some ZnTs confine themselves differently from their normal subcellular localization [38], exhibiting features that are far more intricate than merely effluxing zinc. However, the exact nature of these ZnT subunit interactions and the express purposes of creating functionally redundant ZnT heterodimers in various cell types remain to be elucidated. Thus, further investigation into ZnT dimerization, differential subcellular localizations, and the consequences of subunit interactions should be priorities in future studies of the ZnT protein family.
2.2. ZnTs in Human Diseases
Many diseases and health issues have been linked with the improper function or altered expression of ZnTs (
). More specifically, various ZnTs have been implicated in neurodegenerative diseases and cancers. Both a loss of function [43] and an over-expression of ZnT1 [44] for example, are associated with cancer, but that the former findings may be predictive or prognostic indicator of patient survivability [43]. Mutations in ZnT2 cause zinc concentrations in breast milk to be deficient, which leads to zinc deficiency in children if their primary source of the micronutrient comes from breast milk [45]. Expression levels of ZnT3 are shown to be reduced in post-mortem brains of Alzheimer’s disease (AD) patients [46] and in a mouse model of Mucolipidosis type IV (MLIV) disease [47]. On the other hand, ZnT3 levels appear to be elevated in the cerebellum of a mouse model of AD [48]. Meanwhile, increased expression levels of ZnT6 [18] and decreased expression of ZnT10 [49] have been associated with AD pathology in the hippocampus and frontal cortex, respectively. It is also worth noting that specific mutations within ZnT10 are linked with Parkinson’s disease (PD), dystonia with or without hyper-manganesemia, chronic liver disease, and polycythemia [50][51][52][53]. In prostate cancer, ZnT5 and ZnT6 expression levels are downregulated, while ZnT9 and ZnT10 expression levels are shown to be increased [44]. As mentioned earlier, a mutation in ZnT9 was recently found to be responsible for cerebro-renal syndrome disease [36] and its function may modulate the expression of aggrecan, which is implicated in Temporomandibular joint osteoarthritis [37]. ZnT8 has been widely reported to be involved in DM [34][54][55][56]; however, its role in type II diabetes (T2D), particularly linked with a loss-of-function ZnT8 variant [55], conflicts with evidence supporting treatment of T2D by increasing ZnT8 function [54].
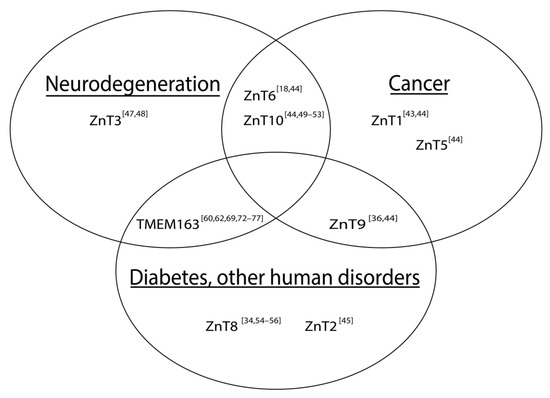
Schematic illustration of associations between altered SLC30 (ZnT) expression and notable human diseases. The Venn diagram includes TMEM163, which has been implicated in certain human diseases. References are represented as numbers in brackets.
The list of zinc transporters that appears to be correlated with various human diseases in the current review is not exhaustive. Nevertheless, it demonstrates that zinc plays many roles in influencing normal and diseased states, and thus, broadening the investigation of these proteins is of significant value to devise or discover a form of therapeutics against many debilitating diseases in humans.
References
- Fukada, T.; Yamasaki, S.; Nishida, K.; Murakami, M.; Hirano, T. Zinc homeostasis and signaling in health and diseases. JBIC J. Biol. Inorg. Chem. 2011, 16, 1123–1134.
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a gatekeeper of immune function. Nutrients 2017, 9, 1286.
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285.
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118.
- Zhang, N.; Duncan, F.E.; Que, E.L.; O’Halloran, T.V.; Woodruff, T.K. The fertilization-induced zinc spark is a novel biomarker of mouse embryo quality and early development. Sci. Rep. 2016, 6, 22772.
- Kim, T.Y.; Hwang, J.J.; Yun, S.H.; Jung, M.W.; Koh, J.Y. Augmentation by zinc of NMDA receptor-mediated synaptic responses in CA1 of rat hippocampal slices: Mediation by Src family tyrosine kinases. Synapse 2002, 46, 49–56.
- Smart, T.G.; Moss, S.J.; Xie, X.; Huganir, R.L. GABAA receptors are differentially sensitive to zinc: Dependence on subunit composition. Br. J. Pharm. 1991, 103, 1837–1839.
- Uchida, K.; Tominaga, M. Extracellular zinc ion regulates transient receptor potential melastatin 5 (TRPM5) channel activation through its interaction with a pore loop domain. J. Biol. Chem. 2013, 288, 25950–25955.
- Sensi, S.L.; Yin, H.Z.; Carriedo, S.G.; Rao, S.S.; Weiss, J.H. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc. Natl. Acad. Sci. USA 1999, 96, 2414–2419.
- Zhao, Y.; Yan, F.; Yin, J.; Pan, R.; Shi, W.; Qi, Z.; Fang, Y.; Huang, Y.; Li, S.; Luo, Y.; et al. synergistic interaction between zinc and reactive oxygen species amplifies ischemic brain injury in rats. Stroke 2018, 49, 2200–2210.
- Gammoh, N.Z.; Rink, L. Zinc in infection and inflammation. Nutrients 2017, 9, 624.
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848.
- Chabosseau, P.; Rutter, G.A. Zinc and diabetes. Arch. Biochem. Biophys. 2016, 611, 79–85.
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534.
- Wong, C.P.; Rinaldi, N.A.; Ho, E. Zinc deficiency enhanced inflammatory response by increasing immune cell activation and inducing IL6 promoter demethylation. Mol. Nutr. Food Res. 2015, 59, 991–999.
- Mohammad, M.K.; Zhou, Z.; Cave, M.; Barve, A.; McClain, C.J. Zinc and liver disease. Nutr. Clin. Pract. 2012, 27, 8–20.
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784.
- Huang, L.; Tepaamorndech, S. The SLC30 family of zinc transporters—A review of current understanding of their biological and pathophysiological roles. Mol. Asp. Med. 2013, 34, 548–560.
- Jeong, J.; Eide, D.J. The SLC39 family of zinc transporters. Mol. Asp. Med. 2013, 34, 612–619.
- Hara, T.; Takeda, T.-A.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017, 67, 283–301.
- Lichten, L.A.; Cousins, R.J. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu. Rev. Nutr. 2009, 29, 153–176.
- Kolaj-Robin, O.; Russell, D.; Hayes, K.A.; Pembroke, J.T.; Soulimane, T. Cation diffusion facilitator family: Structure and function. Febs Lett. 2015, 589, 1283–1295.
- Lu, M.; Fu, D. Structure of the zinc transporter YiiP. Science 2007, 317, 1746–1748.
- Xue, J.; Xie, T.; Zeng, W.; Jiang, Y.; Bai, X.C. Cryo-EM structures of human ZnT8 in both outward- and inward-facing conformations. eLife 2020, 9, e58823.
- Bosomworth, H.J.; Thornton, J.K.; Coneyworth, L.J.; Ford, D.; Valentine, R.A. Efflux function, tissue-specific expression and intracellular trafficking of the Zn transporter ZnT10 indicate roles in adult Zn homeostasis. Metallomics 2012, 4, 771–779.
- Zogzas, C.E.; Aschner, M.; Mukhopadhyay, S. Structural elements in the transmembrane and cytoplasmic domains of the metal transporter SLC30A10 are required for its manganese efflux activity. J. Biol. Chem. 2016, 291, 15940–15957.
- Zogzas, C.E.; Mukhopadhyay, S. Putative metal binding site in the transmembrane domain of the manganese transporter SLC30A10 is different from that of related zinc transporters. Metallomics 2018, 10, 1053–1064.
- Levy, M.; Elkoshi, N.; Barber-Zucker, S.; Hoch, E.; Zarivach, R.; Hershfinkel, M.; Sekler, I. Zinc transporter 10 (ZnT10)-dependent extrusion of cellular Mn(2+) is driven by an active Ca(2+)-coupled exchange. J. Biol. Chem. 2019, 294, 5879–5889.
- Golan, Y.; Alhadeff, R.; Warshel, A.; Assaraf, Y.G. ZnT2 is an electroneutral proton-coupled vesicular antiporter displaying an apparent stoichiometry of two protons per zinc ion. Plos Comput. Biol. 2019, 15, e1006882.
- Guffanti, A.A.; Wei, Y.; Rood, S.V.; Krulwich, T.A. An antiport mechanism for a member of the cation diffusion facilitator family: Divalent cations efflux in exchange for K+ and H+. Mol. Microbiol. 2002, 45, 145–153.
- Ohana, E.; Hoch, E.; Keasar, C.; Kambe, T.; Yifrach, O.; Hershfinkel, M.; Sekler, I. Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J. Biol. Chem. 2009, 284, 17677–17686.
- Sanchez, V.B.; Ali, S.; Escobar, A.; Cuajungco, M.P. Transmembrane 163 (TMEM163) protein effluxes zinc. Arch. Biochem. Biophys. 2019, 677, 108166.
- Valentine, R.A.; Jackson, K.A.; Christie, G.R.; Mathers, J.C.; Taylor, P.M.; Ford, D. ZnT5 variant b is a bidirectional zinc transporter and mediates zinc uptake in human intestinal caco-2 cells. J. Biol. Chem. 2007, 282, 14389–14393.
- Nicolson, T.J.; Bellomo, E.A.; Wijesekara, N.; Loder, M.K.; Baldwin, J.M.; Gyulkhandanyan, A.V.; Koshkin, V.; Tarasov, A.I.; Carzaniga, R.; Kronenberger, K.; et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 Diabetes-associated variants. Diabetes 2009, 58, 2070–2083.
- Weijers, R.N. Three-dimensional structure of β-cell-specific zinc transporter, ZnT-8, predicted from the type 2 diabetes-associated gene variant SLC30A8 R325W. Diabetol. Metab. Syndr. 2010, 2, 33.
- Perez, Y.; Shorer, Z.; Liani-Leibson, K.; Chabosseau, P.; Kadir, R.; Volodarsky, M.; Halperin, D.; Barber-Zucker, S.; Shalev, H.; Schreiber, R.; et al. SLC30A9 mutation affecting intracellular zinc homeostasis causes a novel cerebro-renal syndrome. Brain 2017, 140, 928–939.
- He, D.; Wang, J.; Li, Y.; Wu, G.; Zhu, G.; Chen, L. Low-intensity pulsed ultrasound promotes aggrecan expression via ZNT-9 in temporomandibular joint chondrocytes. Gene 2021, 768, 145318.
- Golan, Y.; Berman, B.; Assaraf, Y.G. Heterodimerization, altered subcellular localization, and function of multiple zinc transporters in viable cells using bimolecular fluorescence complementation. J. Biol. Chem. 2015, 290, 9050–9063.
- Lasry, I.; Golan, Y.; Berman, B.; Amram, N.; Glaser, F.; Assaraf, Y.G. In situ dimerization of multiple wild type and mutant zinc transporters in live cells using bimolecular fluorescence complementation. J. Biol. Chem. 2014, 289, 7275–7292.
- Murgia, C.; Devirgiliis, C.; Mancini, E.; Donadel, G.; Zalewski, P.; Perozzi, G. Diabetes-linked zinc transporter ZnT8 is a homodimeric protein expressed by distinct rodent endocrine cell types in the pancreas and other glands. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 431–439.
- Fukunaka, A.; Suzuki, T.; Kurokawa, Y.; Yamazaki, T.; Fujiwara, N.; Ishihara, K.; Migaki, H.; Okumura, K.; Masuda, S.; Yamaguchi-Iwai, Y.; et al. Demonstration and characterization of the heterodimerization of ZnT5 and ZnT6 in the early secretory pathway. J. Biol. Chem. 2009, 284, 30798–30806.
- Patrushev, N.; Seidel-Rogol, B.; Salazar, G. Angiotensin II requires zinc and downregulation of the zinc transporters ZnT3 and ZnT10 to induce senescence of vascular smooth muscle cells. PLoS ONE 2012, 7, e33211.
- Lehvy, A.I.; Horev, G.; Golan, Y.; Glaser, F.; Shammai, Y.; Assaraf, Y.G. Alterations in ZnT1 expression and function lead to impaired intracellular zinc homeostasis in cancer. Cell Death Discov. 2019, 5, 1–12.
- Singh, C.K.; Malas, K.M.; Tydrick, C.; Siddiqui, I.A.; Iczkowski, K.A.; Ahmad, N. Analysis of zinc-exporters expression in prostate cancer. Sci. Rep. 2016, 6, 36772.
- Kelleher, S.L.; Gagnon, A.; Rivera, O.C.; Hicks, S.D.; Carney, M.C.; Alam, S. Milk-derived miRNA profiles elucidate molecular pathways that underlie breast dysfunction in women with common genetic variants in SLC30A2. Sci. Rep. 2019, 9, 1–13.
- Beyer, N.; Coulson, D.T.; Heggarty, S.; Ravid, R.; Irvine, G.B.; Hellemans, J.; Johnston, J.A. ZnT3 mRNA levels are reduced in Alzheimer’s disease post-mortem brain. Mol. Neurodegener 2009, 4, 53.
- Chacon, J.; Rosas, L.; Cuajungco, M.P. ZnT3 expression levels are down-regulated in the brain of Mcoln1 knockout mice. Mol. Brain 2019, 12, 1–3.
- Zheng, W.; Wang, T.; Yu, D.; Feng, W.Y.; Nie, Y.X.; Stoltenberg, M.; Danscher, G.; Wang, Z.Y. Elevation of zinc transporter ZnT3 protein in the cerebellar cortex of the AbetaPP/PS1 transgenic mouse. J. Alzheimers. Dis. 2010, 20, 323–331.
- Bosomworth, H.J.; Adlard, P.A.; Ford, D.; Valentine, R.A. Altered expression of ZnT10 in Alzheimer’s disease brain. PLoS ONE 2013, 8, e65475.
- Quadri, M.; Federico, A.; Zhao, T.; Guido, C.; Battisti, C.; Delnooz, L.-A.; Severijnen Lara, A.; Mignarri, L.; Monti, A.; Sanna, P.; et al. Mutations in slc30a10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am. J. Hum. Genet. 2012, 90, 467–477.
- Stamelou, M.; Tuschl, K.; Chong, W.K.; Burroughs, A.K.; Mills, P.B.; Bhatia, K.P.; Clayton, P.T. Dystonia with brain manganese accumulation resulting from SLC30A10 mutations: A new treatable disorder. Mov. Disord. 2012, 27, 1317–1322.
- Tuschl, K.; Clayton, P.T.; Gospe, S.M.; Gulab, S., Jr.; Ibrahim, S.; Singhi, P.; Aulakh, R.; Ribeiro, R.T.; Barsottini, O.G.; Zaki, M.S.; et al. Mills. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am. J. Hum. Genet. 2012, 90, 457–466.
- Lambrianides, S.; Nicolaou, P.; Michaelidou, M.; Kakouris, P.; Votsi, C.; Petrou, P.P.; Drousiotou, A.; Minaidou, A.; Demetriou, P.; Voulgaris, C.; et al. A novel SLC30A10 missense variant associated with parkinsonism and dystonia without hypermanganesemia. J. Neurol. Sci. 2020, 418, 117101.
- Chimienti, F.; Devergnas, S.; Pattou, F.; Schuit, F.; Garcia-Cuenca, R.; Vandewalle, B.; Kerr-Conte, J.; Van Lommel, L.; Grunwald, D.; Favier, A.; et al. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J. Cell Sci. 2006, 119, 4199–4206.
- Dwivedi, O.P.; Lehtovirta, M.; Hastoy, B.; Chandra, V.; Krentz, N.A.J.; Kleiner, S.; Jain, D.; Richard, A.-M.; Abaitua, F.; Beer, N.L.; et al. Loss of ZnT8 function protects against diabetes by enhanced insulin secretion. Nat. Genet. 2019, 51, 1596–1606.
- Yi, B.; Huang, G.; Zhou, Z. Different role of zinc transporter 8 between type 1 diabetes mellitus and type 2 diabetes mellitus. J. Diabetes Investig. 2016, 7, 459–465.
