Textile materials, as a suitable matrix for different active substances facilitating their gradual release, can have an important role in skin topical or transdermal therapy. Characterized by compositional and structural variety, those materials readily meet the requirements for applications in specific therapies.
- transdermal therapy
- smart textile
- drug delivery
- stimuli-responsive polymer
1. Introduction
daily routine. The progress of medicine and healthcare has led to a prolonged average life expectancy of modern men. That, however, is constantly increasing peoples’ requirements for information and comfort. Therefore, nowadays, the development of nanotechnology, electronic devices, wireless communication, and information technologies is increasingly entering areas that until recently were considered traditional, difficult to change, and rarely associated with new discoveries. These include some of the oldest man-made materials, such as textiles. Today, the word design is more relevant to them than ever before, because smart textiles have a great future utilization in healthcare [1][2][3], medicine [4][5][6][7][8], transport [9][10], sports and leisure [11][12], safety and personal protective equipment [13], construction [14], interior design [15][16], agriculture [17], sensors and biosensors [18][19][20], etc. Materials creation is associated with combining their known properties with new functionality to ensure active interaction with the environment, i.e., the ability to react and adapt to changes [21].
Textile materials used for medical purposes are structures and products that are used in first aid, in clinical and hygienic practice. This usage in various forms dates back to ancient times. For example, natural materials such as silk, cotton, linen, etc., have been applied as dressings for wounds and sutures. In the 20th century, new in vivo application of biomedical textiles was established in the cardiovascular, digestive, excretory, etc., systems following the introduction of artificial and synthetic fibers. Today, the new challenges are focused in the field of regenerative and tissue engineering, in systems for delivery of biologically active substances (BAS), in the creation of intelligent textile materials for monitoring and treatment of various health conditions [22][23].
Currently, the most commonly used methods for BAS delivery in the human body are: peroral; intravenously; inhalation; etc. In recent years, advances in drug delivery systems have given new impetus to another long-established mode of prevention and treatment, namely transdermal therapy. It facilitates effects upon a number of nutritional deficiencies that leads to problems in the immune, hormonal, and nervous systems, to protect cells from oxidative destruction, to influence the formation of tumors, and therapy in patients with diabetes. Textiles modified with a stimuli-responsive drug carrier have great potential to answer to the specific patient case and can be used as both topical and systemic (transdermal) drug delivery systems [24]. Aromatherapy [25], antimicrobials [26] and painkillers [27], hormone therapy [28], treatment of acne [29] psoriasis [30], atopic dermatitis [31][32], melanoma [33], etc., are just some of the areas where textiles are involved as an important functional element of systems for transdermal therapy, wound dressings, dermatology, etc. The utilization of biomedical textiles in dermatology and transdermal therapy is characterized by easy application, greater comfort, and less pain for the patients, their shorter hospitalization, etc. [34][35].
Several excellent review articles describing applications of textile materials and different pharmaceutical nanocariers in topical and transdermal drug delivery have been published [36][37][38]. The increased number of studies in this field is as a result of the current interest in the development of the platform for continuous monitoring and assisting the health status of individuals via telemedicine [39] and personalized medicine [40]. This modern concept for continuous personalized patient care influences the advance of wearable textiles that allow administering a predetermined drug dose at certain intervals. As the processes taking place in wound healing or transdermal therapy are complex, the design of delivery systems for BAS is dependent on a number of multi-stage, interconnected factors. One of the crucial factors to be considered is the ability of known nanocarriers to respond to external stimuli so that the delivery to the target and release control be more precise.
2. Skin Structure. Mechanism of the Transdermal System for BAS Delivery. Factors Determining Its Effectiveness
2
stratum corneum, acts as a major barrier to the absorption of BAS. It is a lipophilic membrane of which thickness varies from 0.06 mm in the eyelids to 0.8 mm in the skin of the heels [41]. The dermis is the second deepest skin layer and is composed mainly of connective tissue. It is rich in blood vessels, through which the oxygen and nutrients necessary for its vital activity reach the skin. The subcutaneous tissue is under the dermis and is composed mainly of adipose tissue, with varying severity in different parts of the body. This layer helps regulate the temperature.
stratum corneum. It is 10–20 μm thick [42]. It consists of flat cells made up of keratin, which have turned into overlapping scales, arranged in many deposits (on the feet more than 100) embedded in a lipid matrix consisting mainly of ceramides, cholesterol, and free fatty acids. The
stratum corneum is water-resistant, while acidic solutions and basic solutions make it swell and become permeable [43][44].
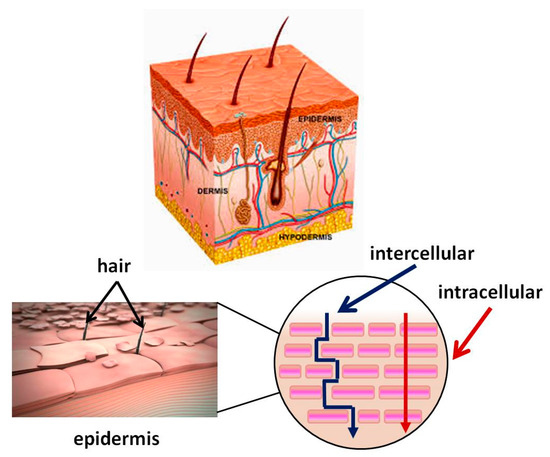
Figure 1. Skin structure. Mechanism of passage through the skin of biologically active substance BAS.
The skin condition difference, the integrity of its layers, the type of disease, and the necessary treatment are some of the factors that determine the technology for creating pharmaceutical materials. In transdermal therapy, BAS must pass through all layers of the skin to reach the systemic circulation. In the topical skin treatment, BAS aim at treating the skin itself and its layers on a particular affected body area (epidermis and dermis) [45]. In this case, the normal skin structure and function may be preserved or disrupted to a greater or lesser extent. The skin injury is known as a wound. According to the number of affected skin layers, wounds are: a superficial wound (only epidermal layer is affected); a partial thickness wound with injures, involving both the epidermis and the deeper dermal layers, the blood vessels, sweat glands, and hair follicles, inclusive; full-thickness wounds, the underlying subcutaneous fat or deeper tissues are damaged in addition to the epidermis and dermal layer [46].
stratum corneum are intercellularly, intracellularly, and via the follicles. The intercellular pathway predominates over the intracellular [45]. Hydrophobic drugs penetrate skin intercellular. They dissolve and diffuse through the non-aqueous lipid matrix of the
stratum corneum [47]. Hydrophilic molecules pass through the intracellular pathway penetrating through the corneocyte cells. However, the molecules need to pass through the intercellular lipids to reach the next corneocyte. Therefore, a part of their pathway is partially intercellular and may be decisive for the diffusion rate.
stratum corneum by both routes [48]. Low molecular weight (MW < 500 Da) and high pharmacological potency [49] are other drug molecules characteristics important for a transdermal therapy.
The third possible route for hydrophilic compounds to reach the dermis is through defects in the skin structure—for instance, through hair follicles, sweat glands, etc. [50]. The transfer through the follicle is considered insignificant, as according to data, the part of the skin covered with hair is quite small—only 0.1%.
stratum corneum
3. Biofunctional Textile Materials and Their Preparative Methods
The development and use of biofunctional textiles is a rapidly developing interdisciplinary field of science and application, due to the diverse possibilities of use and growing demand. The contact of the material with human skin is essential, so the new properties obtained must be effective, but also provide comfort to the user. These textiles include materials with antimicrobial properties, those that supply BAS or absorbents for substances released during sweating and leading to an unpleasant odor, etc.
The manufacturing process of biofunctional textile materials consists of fibers production using different natural or synthetic polymers and yarn processing by various engineering methods. The next step is surface modification via different chemical, physical, and biological treatment [51]. Biofunctional fabrics combine conventional textiles with modern advances in the development of drug delivery systems, as well as the application of new approaches to the transdermal application of bioactive molecules [52][53][54][55]. Direct immobilization of the active compound on the fabric surface can lead to some disadvantages as a change in its biological activity, controllability, and problems with skin interaction (
Figure 2B) [53][55]. Other methods involve surface modification of textile materials and introduction of new functional groups, as well as implementation of polymeric binders for immobilization or encapsulation of various organic and inorganic micro- and nanoparticles (
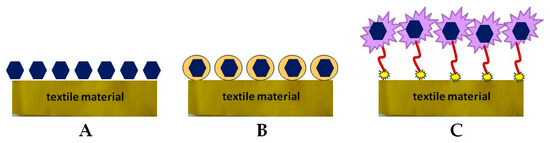
Figure 2.
A
B
C
Biofunctional textile materials can be produced by traditional techniques: exhaustion from solution; irrigation; spraying; pad-dry-cure; using sol-gel or layer-by-layer technique [51][56]. They can be classified according to their chemical composition, structure, and the way drug carriers attach. The materials used in medical practice can be produced from only one fiber type or from different types of fibers. The choice depends on the desired specific characteristics of the obtained material, which combine the medical purposes with appropriate functional properties, providing comfort for the patient [57]. For example, viscose/polyester dressings integrate the properties of viscose as a facile modification with those of drug carriers: high absorbency, breathability, comfort, and softness with the resistance of wrinkle, tear, and microbial attacks of strong polyester fibers [58]. The individual fibers or threads can be loaded or modified with drug carriers. After that, they are assembled into a flexible wound dressing or other biofunctional material through textile engineering processes. This approach was used to prepare cotton threads coated with a layer of electrically conductive ink and covered with a hydrogel layer of alginate/poly(ethylene glycol) diacrylate carrying thermoresponsive particles. The fibers were then assembled into fabrics for delivering antibiotics and vascular endothelial growth factor [59].
The structure of fabrics can be woven, knitted, and nonwoven. The woven fabric can be modified with bioactive complex that is physically absorbed or adsorbed, coated, encapsulated or covalently conjugated on the fabric [60]. Nonwoven medical textiles have highly porous structure and adequate compression behavior that enhance the healing rate by controlled bioactive agent delivery, transport of nutrients, cellular migration, and metabolic wastes to allow the regeneration and the formation of new tissue [61]. The diameter of the fibers and the structure of the knitted fabric have been shown to play an important control role in the drug-releasing properties of the material [62]. The interaction of the transdermal delivery system with all conventional textile materials leads to the production of wearable devices for the delivery of drugs or biofunctional clothing.
The stimuli-responsive polymers can be attached to the textile surface by physical or chemical bonds. The physical adsorption can be used when the aim is to release the whole bioactive complex from stimuli-responsive polymer and conjugated BAS. In this case, the stimuli trigger the disruption of low energy physical bonds. When the corresponding stimulus-responsive polymer is bonded covalently to the functional groups of the fibers, the role of the applied stimuli is to change the structure of the polymer and to stimulate the release of BAS alone.
New treatment methods—low temperature plasma, corona, light curing, and photopolymerization—have been applied successfully to improve the interaction of stimuli-responsive polymers with fiber surfaces. These methods produce effects in a depth of a few nanometers and can be used for changing the surface specifics of textile fibers without any change in bulk properties [63].
4. Stimuli-Responsive Drug Carrier for Delivering Bioactive Substances
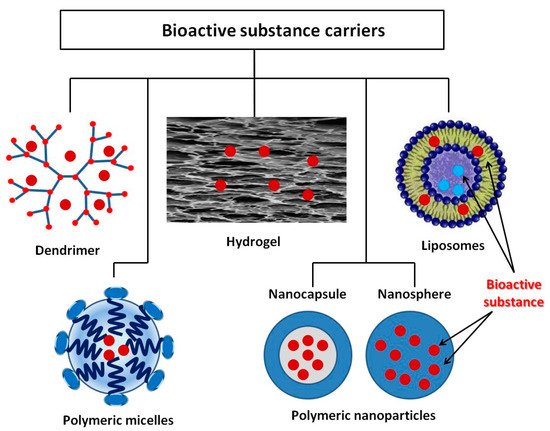
Microencapsulation is a process by which the active substance is coated with a polymeric material called a shell. Microcapsules immobilized onto textile materials find various applications, including the supply of BAS. In recent years, however, active studies have been carried out on other structures, which have shown very good properties with regard to in vivo delivery of drugs that is promising for their in vitro application. The review by M.R. ten Breteler et al. [64] deals with implementation of textile materials modified with cyclodextrins, aza-crown ethers, fullerenes, ion exchange or hollow fibers, as well as with nanoparticles used as BAS carriers for treating acne, psoriasis, atopic dermatitis, melanoma, etc. [65]. Massella et al. expand the possible drug carriers for textile modification with nanospheres, micro- and nanocapsules, liposomes, inorganic particles, and micro-hydrogel [36]. In addition to the above structures, dendrimers are other interesting polymeric forms for biofunctional tissue production [66]. The present review describes some of vanguard polymeric formations as dendrimers, polymer micelles, liposomes, and various polymer nanocomposites, as well as crosslinked polymers in the form of hydrogels (Figure 3). What is common of the considered systems for BAS delivery is their intelligence (ability to react to various internal and external influences) which allows controlled BAS release. Thus, BAS use for application to the skin and in transdermal therapy becomes safer and more successful. The release mechanism of these systems includes desorption of BAS, diffusion through the carrier matrix, erosion, biodegradation, etc. Stimuli-responsive polymers are “intelligent” materials that can respond to a different small external stimulus with a change in their basic properties. They can be classified according to their nature as physical (temperature, ultrasound, mechanical stress, irradiation with light, magnetic and electrical fields), chemical (variation of pH and ions, dipole-dipole interaction, polarity of environment), and biological (BAS, biomolecules, proteins, enzymes) [67][68]. In shape, these materials can be linear, cross-linked, or branched.
4. Stimuli-Responsive Drug Carrier for Delivering Bioactive Substances
Microencapsulation is a process by which the active substance is coated with a polymeric material called a shell. Microcapsules immobilized onto textile materials find various applications, including the supply of BAS. In recent years, however, active studies have been carried out on other structures, which have shown very good properties with regard to in vivo delivery of drugs that is promising for their in vitro application. The review by M.R. ten Breteler et al. [67] deals with implementation of textile materials modified with cyclodextrins, aza-crown ethers, fullerenes, ion exchange or hollow fibers, as well as with nanoparticles used as BAS carriers for treating acne, psoriasis, atopic dermatitis, melanoma, etc. [68]. Massella et al. expand the possible drug carriers for textile modification with nanospheres, micro- and nanocapsules, liposomes, inorganic particles, and micro-hydrogel [36]. In addition to the above structures, dendrimers are other interesting polymeric forms for biofunctional tissue production [69]. The present review describes some of vanguard polymeric formations as dendrimers, polymer micelles, liposomes, and various polymer nanocomposites, as well as crosslinked polymers in the form of hydrogels (Figure 3). What is common of the considered systems for BAS delivery is their intelligence (ability to react to various internal and external influences) which allows controlled BAS release. Thus, BAS use for application to the skin and in transdermal therapy becomes safer and more successful. The release mechanism of these systems includes desorption of BAS, diffusion through the carrier matrix, erosion, biodegradation, etc. Stimuli-responsive polymers are “intelligent” materials that can respond to a different small external stimulus with a change in their basic properties. They can be classified according to their nature as physical (temperature, ultrasound, mechanical stress, irradiation with light, magnetic and electrical fields), chemical (variation of pH and ions, dipole-dipole interaction, polarity of environment), and biological (BAS, biomolecules, proteins, enzymes) [70,71]. In shape, these materials can be linear, cross-linked, or branched.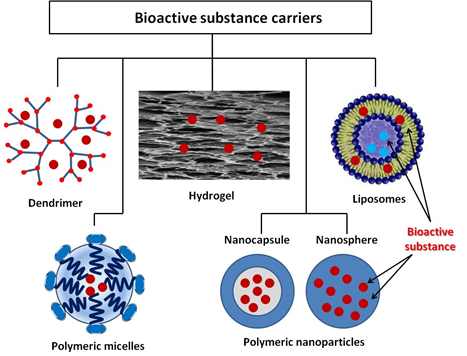
Figure 3. BAS delivery carriers.
5. Multifunctional and Intelligent Textiles—Perspectives and Expectations
The development of systems for the delivery of therapeutic compounds and in particular for transdermal therapy is associated with the widespread use of biomacromolecules from renewable sources and natural origin (plant or animal). These are proteins (collagen, keratin, fibroin, etc.), lipoproteins, and polysaccharides (chitosan, cyclodextrin, hyaluronic acid, heparin, pectin, etc.). Their advantages compared to synthetic compounds are non-toxicity, non-immunogenicity, good biocompatibility. They have different functional groups (hydroxyl, amino, and carboxyl), which can be modified and thus be permanently linked to the textile materials.
The development of biofunctional textile materials that release BAS in a controlled manner, allowing monitoring the process via a change in their properties is an example of creating new intelligent materials. Those materials can react to various physical, chemical, or biological environment factors (e.g., temperature, pH, light, magnetic field, solvent, etc.). Other impacts are the appearance or increase in the concentration of various biological products (enzymes, elevated glucose levels, etc.) [69][70]. The design of such materials should combine their multifunctional action with monitoring the health status and/or the effects of various environmental stimuli, followed by an appropriate response associated with a change in their properties, to the delivery of BAS, to antimicrobial and other reactions related to supporting the healing process.
References
- Mečņika, V.; Hoerr, M.; Krieviņš, I.; Schwarz, A. Smart textiles for healthcare: Applications and technologies.
- Rural Environ. Educ. Personal. REEP
- 2014
- , 7, 150–161.
- Boesel, L.F.; Furundžić, D.P.; Furundžić, N.Z.; Gedanken, A.; Grabchev, I.; Haj Taieb, A.; Ivanoska-Dacik, A.; Malionowski, S.; Marković, D.; Mohr, G.; et al. Smart Textiles for Healthcare and Medicine Applications (WG1): State-of-the Art Report, CONTEXT Project; University College Cork: Cork, Ireland, 2020.
- Zhong, W. An Introduction to Healthcare and Medical Textiles; DEStech Publications Inc.: Lancaster, PA, USA, 2013.
- Grabchev, I.; Staneva, D.; Vasileva-Tonkova, E.; Alexandrova, R. Surface functionalization of cotton fabric with fluorescent dendrimers, spectral characterization, cytotoxicity, antimicrobial and antitumor activity. Chemosensors2019, 7, 17.
- Malis, D.; Jeršek, B.; Tomšič, B.; Štular, D.; Golja, B.; Kapun, G.; Simončič, B. Antibacterial activity and biodegradation of cellulose fiber blends with incorporated ZnO. Materials2019, 12, 3399.
- Staneva, D.; Vasileva-Tonkova, E.; Bosch, P.; Grozdanov, P.; Grabchev, I. Synthesis and characterization of a new PAMAM metallodendrimer for antimicrobial modification of cotton fabric. Res.2018, 26, 332–340.
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine2015, 5, 22.
- Van Langenhove, L. Smart Textiles for Medicine and Healthcare: Materials, Systems and Applications; Woodhead Publishing: Cambridge, UK, 2007.
- Jerkovic, I.; Grancaric, A.M.; Koncar, V. Structural health monitoring of composites with newly developed textile sensors in situ. IOP Conf. Ser. Mater. Sci. Eng.2018, 460, 012046.
- Wagner, M. Multidisciplinary Know-How for Smart-Textiles Developers; Woodhead Publishing: Cambridge, UK, 2013; pp. 444–467.
- Shishoo, R. Textiles in Sportswear, 1st ed.; Woodhead Publishing: Cambridge, UK, 2015.
- Chittenden, T. Skin in the game: The use of sensing smart fabrics in tennis costume as a means of analyzing performance. Text.2017, 4, 22.
- Dolez, P.I.; Mlynarek, J. Smart materials for personal protective equipment. In Smart Textiles and Their Applications; Koncar, V., Ed.; Woodhead Publishing: Cambridge, UK, 2016.
- Oliveira, A. Smart textile for architecture: Living with technology. In Proceedings of the 2nd International Conference on Human Interaction and Emerging Technologies: Future Applications (IHIET—AI 2020), Lausanne, Switzerland, 23–25 April 2020.
- Qureshi, A.K. Utilizing smart textiles in interior design to replace conventional architectural finishes. TEXTEH Proc.2019, 105–109.
- Youssef, M.M. Smart textiles as hybrid interactive materials A responsive behaviour towards transformable surfaces. Des. J.2017, 7, 215–226.
- Thakur, S. Shape memory polymers for smart textile applications. In Textiles for Advanced Applications; Kumar, B., Ed.; IntechOpen: London, UK, 2017.
- Staneva, D.; Grabchev, I. Heterogeneous sensors for ammonia, amines and metal ions based on a dendrimer modified fluorescent viscose fabric. Dyes Pigment.2018, 155, 164–170.
- Micus, S.; Haupt, M.; Gresser, G.T. Automatic joining of electrical components to smart textiles by ultrasonic soldering. Sensors2021, 21, 545.
- Keum, K.; Heo, J.S.; Eom, J.; Lee, K.W.; Park, S.K.; Kim, Y.-H. Highly sensitive textile-based capacitive pressure sensors using PVDF-HFP/ionic liquid composite films. Sensors2021, 21, 442.
- Hadjiev, H.; Rrahnev, I.; Philippov, P. Hybrid microelectronic technology in textile techniques with the aim of realizing smart textiles. In Proceedings of the 38th International Spring Seminar on Electronics Technology (ISSE), Eger, Hungary, 6–10 May 2015; pp. 75–79.
- Qin, Y. Medical Textile Materials; Woodhead Publishing: Cambridge, UK, 2016.
- Jiao, Y.; Li, C.; Liu, L.; Wang, F.; Liu, X.; Mao, J.; Wang, L. Construction and application of the textile-based tissue engineering scaffold: A review. Sci.2020, 8, 3574–3600.
- van Langenhove, L. Advances in Smart Medical Textiles, Treatments and Healt Monitoring; Woodhead Publishing: Cambridge, UK, 2016.
- West, A.J.; Annett-Hitchcock, K.E. A critical review of aroma therapeutic applications for textiles. JTATM2014, 9, 1–13.
- Hashemikia, S.; Hemmatinejad, N.; Ahmadi, E.; Montazer, M. Antibacterial and anti-inflammatory drug delivery properties on cotton fabric using betamethasoneloaded mesoporous silica particles stabilized with chitosan and silicone softener. Drug Deliv.2016, 23, 2946–2955.
- Zurita, R.; Puiggalí, J.; Rodríguez-Galán, A. Loading and release of ibuprofen in multiand monofilament surgical sutures. Biosci.2006, 6, 767–775.
- Kopper, N.W.; Gudeman, J.; Thompson, D.J. Transdermal hormone therapy in postmenopausal women: A review of metabolic effects and drug delivery technologies. Drug Des. Dev. Ther.2008, 2, 193–202.
- Atabay, N.; Sariişik, A.M.; Karavana, S.Y.; Rençber, S. A novel plaster containing benzoyl peroxide microsponges: Formulation, development and evaluation. Ind. Text.2020.
- Radu, C.D.; Cerempei, A.; Salariu, M.; Parteni, O.; Ulea, E.; Campagne, C. The potential of improving medical textile for cutaneous diseases. IOP Conf. Ser. Mater. Sci. Eng.2017, 254, 062010.
- Palmer, B.C.; DeLouise, L.A. Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules2016, 21, 1719.
- Wang, W.; Hui, P.C.L.; Kan, C.-W. Functionalized textile based therapy for the treatment of atopic dermatitis. Coatings2017, 7, 82.
- Naves, L.B.; Dhand, C.; Venugopal, J.R.; Rajamani, L.; Ramakrishna, S.; Almeida, L. Nanotechnology for the treatment of melanoma skin cancer. Biomater.2017, 6, 13–26.
- Volkmar, T.B. Biofunctional Textiles and the Skin, 1st ed.; Hipler, U.C., Elsner, P., Burg, G., Itin, P.H., Eds.; Kanger Publishers: Basel, Switzerland, 2006; pp. 51–66.
- Schoe llhammer, C.; Blankschtein, D.; Langer, R. Skin permeabilization for transdermal drug delivery: Recent advances and future prospects. Opin. Drug Deliv.2014, 11, 393–407.
- Massella, D.; Argenziano, M.; Ferri, A.; Guan, J.; Giraud, S.; Cavalli, R.; Barresi, A.A.; Salaün, F. Bio-functional textiles: Combining pharmaceutical nanocarriers with fibrous materials for innovative dermatological therapies. Pharmaceutics2019, 11, 403.
- Chatterjee, S.; Hui, C.L. Review of stimuli-responsive polymers in drug delivery and textile application. Molecules2019, 24, 2547.
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug Delivery Systems and Materials for Wound Healing Applications. Drug Deliv. Rev.2018, 127, 138–166.
- Hernandez, C.; Mallow, J.; Narsavage, G.L. Delivering telemedicine interventions in chronic respiratory disease. Breathe2014, 10, 199–212.
- Yetisen, A.K.; Martinez-Hurtado, J.L.; Ünal, B.; Khademhosseini, A.; Butt, H. Wearables in medicine. Mater.2018, 30, 1706910.
- Lis, M.J.; Carmona, Ó.G.; Carmona, C.G.; Bezerra, F.M. Inclusion complexes of citronella oil with β-cyclodextrin for controlled release in biofunctional textiles. Polymers2018, 10, 1324.
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. J. Pharmacol.2015, 172, 2179–2209.
- Margetts, L.; Sawyer, R. Transdermal drug delivery: Principles and opioid therapy. Educ. Anaesth. Crit. Care Pain2007, 7, 171–176.
- Hu, J.; Liu, W.; Liu, B. Fabric-Supported Chitosan Modified Temperature Responsive PNIPAAm/PU Hydrogel and the Use Thereof in Preparation of Facial Mask. U.S. Patent 7,780,979, 24 August 2010.
- Elias, P. Epidermal lipids, barrier function, and desquamation. Investig. Dermatol.1983, 80, 44.
- Pilgram, G.; Engelsma-van Pelt, A.; Bouwstra, J.; Koerten, H. Electron diffraction provides new information on human stratum corneum lipid organization studied in relation to depth and temperature. Investig. Dermatol.1999, 113, 403–409.
- Bouwstra, J.; Gooris, G.; Ponec, M. Skin lipid organization, composition and barrier function. IFSCC Mag.2007, 10, 297–307.
- Barry, B. Drug delivery routes in skin: A novel approach. Drug Deliv. Rev.2002, 54, 31–40.
- Cannon, J.B. Lipids in transdermal and topical drug delivery. Pharm. Rev.2014, 17, 7.
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. Pharm. Sci.2008, 97, 2892–2923.
- Tanwar, H.; Sachdeva, R. Transdermal drug delivery system: A review. IJPSR2016, 7, 2274–2290.
- Wiedersberg, S.; Guy, R.H. Transdermal drug delivery: 30 years of war and still fighting! Control. Release2014, 190, 150–156.
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics2015, 7, 438–470.
- Hipler, U.; Elsner, P. Biofunctional textiles and the skin. In Current Problems in Dermatology, 1st ed.; Burg, G., Ed.; S. Karger AG: Basel, Switzerland, 2006; Volume 33, pp. 1–204.
- Hu, J. Active Coatings for Smart Textiles; Woodhead Publishing: Cambridge, UK, 2016.
- Mihailiasa, M.; Caldera, F.; Li, J.; Peila, R.; Ferri, A.; Trotta, F. Preparation of functionalized cotton fabrics by means of melatonin loaded β-cyclodextrin nanosponges. Polym.2016, 142, 24–30.
- Ruela, A.; Perissinato, A.; de Sousa Lino, M.; Mudrik, P.; Pereira, G. Evaluation of skin absorption of drugs from topical and transdermal formulations. J. Pharm. Sci.2016, 52, 527–544.
- Roberts, M.; Mohammed, Y.; Pastore, M.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.; Abd, E.; Leite-Silva, V.; Benson, H.; et al. Topical and cutaneous delivery using nanosystems. Control. Release2017, 247, 86–105.
- Joshi, S.; Jalalpure, S.; Kempwade, A.; Peram, M. Fabrication and in-vivo evaluation of lipid nanocarriers based transdermal patch of colchicine. Drug Deliv. Sci. Technol.2017, 41, 444–453.
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater.2017, 9, 435.
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurface Biotribol.2015, 1, 161–176.
- El Ghoul, Y.; Salah, F.; Majdoub, H.; Sakli, F. Synthesis and study of drug delivery system obtained via b-cyclodextrin functionalization of viscose/polyester dressings. Ind. Text.2017, 47, 489–504.
- Mostafalu, P.; Kiaee, G.; Giatsidis, G.; Khalilpour, A.; Nabavinia, M.; Dokmeci, M.R.; Sonkusale, S.; Orgill, D.P.; Tamayol, A.; Khademhosseini, A. A textile dressing for temporal and dosage controlled drug delivery. Funct. Mater.2017, 27, 1702399.
- Singh, J. Nonwoven: A versatile fabric. Sci. Eng.2014, 4, 5.
- Marcincin, A. Modification of fiber-forming polymers by additives. Polym. Sci.2002, 27, 853–913.
- Massella, D.; Ancona, A.; Garino, N.; Cauda, V.; Guan, J.; Salaun, F.; Barresi, A.A.; Ferri, A. Preparation of bio-functional textiles by surface functionalization of cellulose fabrics with caffeine loaded nanoparticles. IOP Conf. Ser. Mater. Sci. Eng.2018, 460, 012044.
- Ten Breteler, M.; Nierstrasz, V.; Warmoeskerken, M. Textile slow-release systems with medical applications. AUTEX Res. J.2002, 2, 175–189.
- Korting, H.; Schäfer-Korting, M. Carriers in the topical treatment of skin disease. In Handbook of Experimental Pharmacology; Springer: New York, NY, USA, 2010; Volume 197, pp. 435–468.
- Wolinsky, J.; Grinstaff, M. Therapeutic and diagnostic applications of dendrimers for cancer treatment. Drug Deliv. Rev.2008, 9, 1037–1055.
- Roy, I.; Gupta, M.N. Smart polymeric materials: Emerging biochemical applications. Biol.2003, 10, 1161–1171.
- Stuart, M.A.C.; Huck, W.T.S.; Minko, S. Emerging applications of stimuli-responsive polymer materials. Mater.2010, 9, 101–111.
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-responsive polymeric nanocarriers for drug delivery, imaging, and theragnosis. Polymers2020, 12, 1397.
- Morey, M.; Pandit, A. Responsive triggering systems for delivery in chronic wound healing. Drug Deliv. Rev.2018, 129, 169–193.
