The global warming and the dangerous climate change arising from the massive emission of CO2 from the burning of fossil fuels have motivated the search for alternative clean and sustainable energy sources. However, the industrial development and population necessities make the decoupling of economic growth from fossil fuels unimaginable and, consequently, the capture and conversion of CO2 to fuels seems to be, nowadays, one of the most promising and attractive solutions in a world with high energy demand. In this respect, the electrochemical CO2 conversion using renewable electricity provides a promising solution. However, faradaic efficiency of common electro-catalysts is low, and therefore, the design of highly selective, energy-efficient, and cost-effective electrocatalysts is critical. Carbon-based materials present some advantages such as relatively low cost and renewability, excellent electrical conductivity, and tunable textural and chemical surface, which show them as competitive materials for the electro-reduction of CO2.
- carbon dioxide
- electro-reduction
- carbon-based materials
- value-added products
1. Introduction
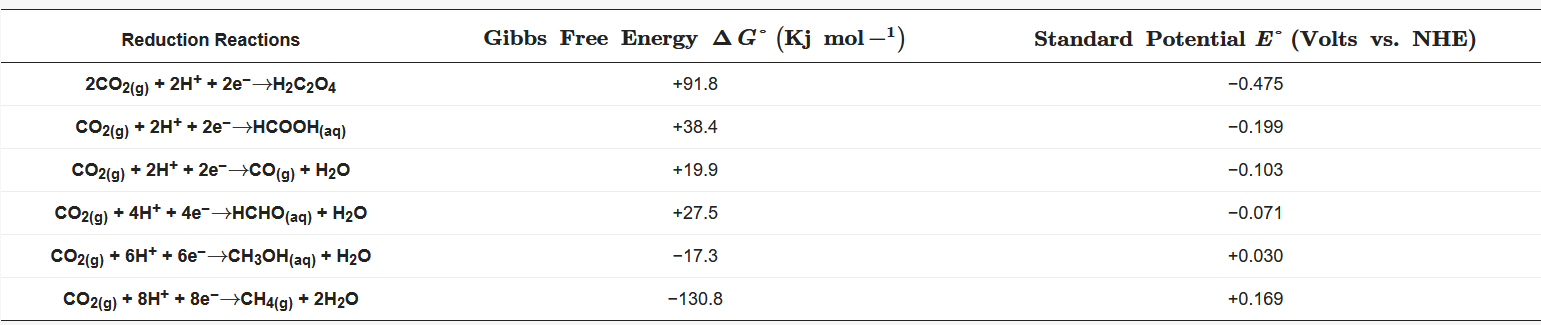
The energy supply currently depends mostly on fossil fuels, causing a continuous accumulation and, therefore, an excess of CO2 in the atmosphere, bringning negative effects on the environment. The population and live standards growth make nor imaginable the decoupling of energy supply from fossil fuels. Faced with this situation, different altenatives have been proposed to mitagate the enviromental impact and dependence on nonrenewable energy sources. The conversion of CO2 into value-added products by chemical reactions seems to be the most promising and attractive solution since, together with the reduction of the atmospheric CO2 levels, CO2 is efficiently recycled stablishing an ideal zero-emission carbon balance. CO2 can be converted to added-value products by photochemical [1][2][3][4], thermochemical [5][6][7][8], radiochemical [9] [10], biochemical [11][12][13][14], and electrochemical strategies [15][16][17][18]. However, the most interesting alternative is the capture and use of CO2 as raw material to produce various products ( Table 1) through its electrochemical reduction since this is a flexible and controllable process with mild and safe operating conditions and low equipment cost, which also allows coupling environmentally friendly non-fossil energy from renewable sources. Taking into account these advantages, many efforts have been made worldwide in the development and improvement of the technology available for CO2 electro-conversion.
Table 1. Equilibrium potential and Gibbs free energy for CO2 reduction reactions.
However, despite the fact that electro-reduction of CO2 (CO2RR) is thermodynamically viable, its transformation presents very slow reaction kinetics and usually requires significant energy expenditure [19] due to the high stability and inertness of the CO2 molecule [20]. Therefore, an extensive research has been developed by the overall scientific community focused on the electrocatalyst design, since the efficiency and selectivity of the reduction reaction is strongly dependent on the electrode nature, properties, and configuration [21]. An ideal catalyst for CO2 electroreduction requires: (i) Being able to mediate the transfer of electrons coupled to protons, (ii) having a low over potential for the activation of the CO2 molecule, (iii) exhibiting a selectivity preferably towards a target product, and (iv) preserving structural integrity during prolonged operation.
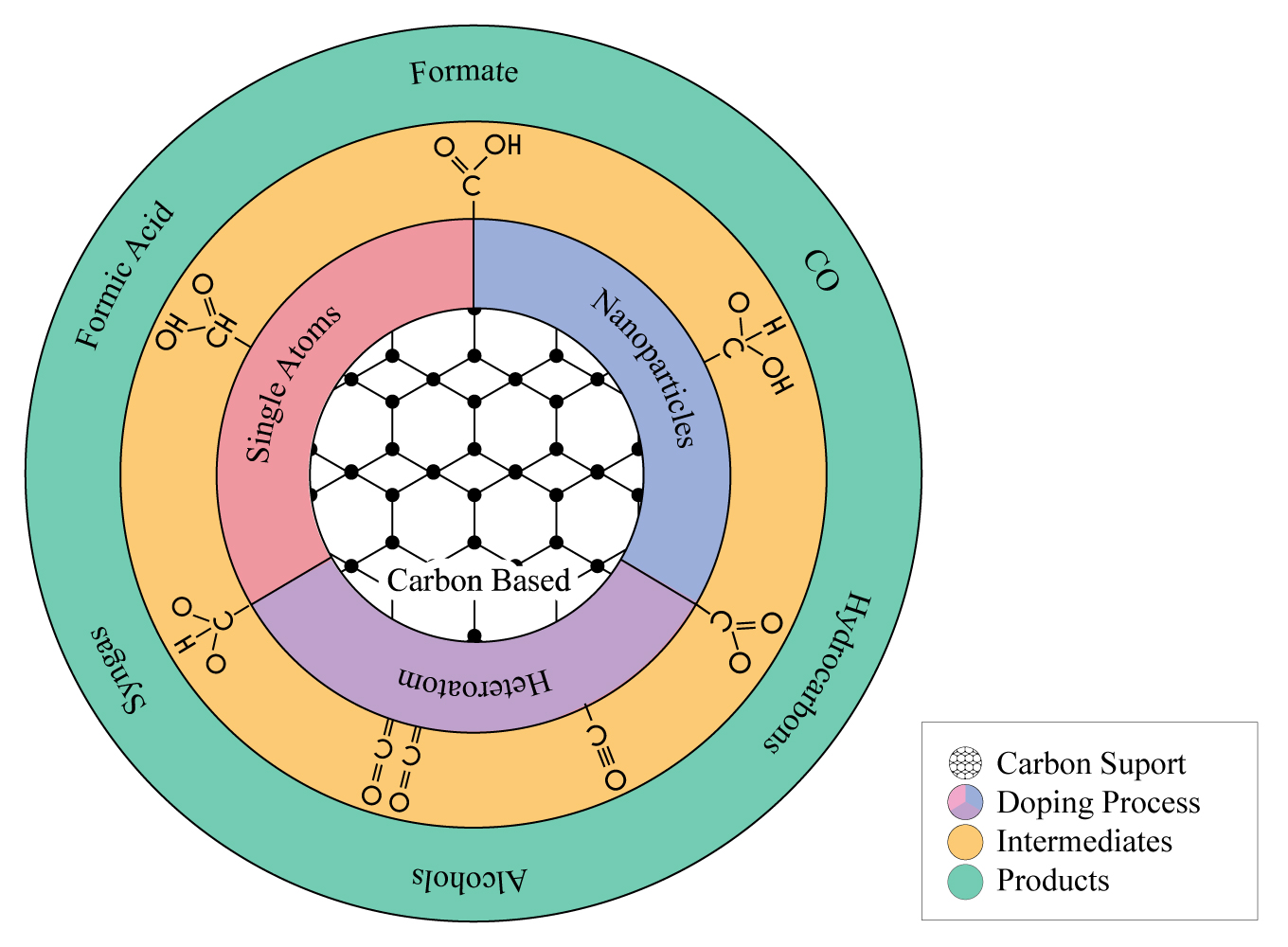
Lately, carbon-based catalysts have attracted much attention due to their relatively low cost and renewability, good chemical stability, excellent electrical conductivity, tunable textural and chemical surface, and large surface area, containing micropores, mesopores, and macropores that favor adsorption, access, and diffusion of molecules to the internal active sites of the material [22]. Due to these particular characteristics, carbon-based materials have been extensively used as electrocatalysts for CO2 reduction either as supports to disperse different metallic particles with several sizes (single-atoms, dual-atoms, nanoparticles) or as direct catalyst by functionalization with heteroatoms to prepare economical and sustainable metal-free electro-catalysts [23] . ( Figure 1 ).
Figure 1. Value-added products using carbon- based catalysts.
2. Metal-Free Carbon Materials as Catalyst
Metal-free carbon-based catalysts emerge as an alternative to overcome the difficulties that arise when using metals as catalysts, such as their limited availability and poor durability that prevent their application on large scales. However, the activity of carbon materials itself is poor, so heteroatoms (N, B, S, P, F) are introduced into the carbon structure to promote electrocatalytic activity and selectivity [24]. Carbon doping with foreign heteroatoms affects the electronic structure of carbon materials since the different size and electronegativity of such foreign atoms compared to carbon atoms lead to a charge redistribution and, consequently, modify their electrochemical catalytic properties [25]. Additionally, the covalent chemical bonds between the carbon and the doped atoms avoid segregation problems occurring in metal-based catalysts leading to better operational stability [25]. Different heteroatoms have been used to dope carbon obtaining materials with good electrochemical performance in the CO2 reduction, and among them, N and B have been the most studied.
2.1. N-Doped Carbon-Based Materials
The N atom has a similar size to the C atom but higher electronegativity [26], therefore the defects caused by nitrogen doping can break the electroneutrality of C atoms in the hexagonal carbon structure [27] leading to an enhanced electronic/ionic conductivity without distortion in the local geometry that influences the electrocatalytic activity [28]. N-doped carbons have shown to be promising candidates as catalysts for the electro-reduction of CO2 due to the low over-potentials obtained, the high activity, stability, and selectivity towards certain products ascribed to this surface properties modification. The nitrogen species are located in several places within the carbon skeleton, which results in different active sites. The electrocatalytic behavior of the N-doped carbons towards CO2RR is deeply dependent on the type of nitrogenated surface group and its content [26].
2.1.1. N-Doping Methodology and N-Doped Catalyst Active Sites
Different carbon and nitrogen precursors have been used for the synthesis of N-doped carbon electrocatalysts (Table 2). Two main doping strategies have been developed: In situ and post-doping treatments. The first consists of simultaneously perform both the synthesis and doping of carbon-based materials at the same time; while in the second, the carbon material is first synthesized and then doped in a subsequent process [22][25]. After doping, four types of nitrogen species can be identified in the carbon skeleton by XPS: Pyridinic (398.5 eV), pyrrolic/pyridonic (399.9 eV), quaternary or graphitic N (401.0 eV), and oxidized pyridinic species (403.4 eV) [29][30]. The total amount of nitrogen fixed on the carbon structure and the nature of N functionalities clearly depends on the N precursor source, doping methodology, and carbon material.
Table 2. Carbon and nitrogen precursors to construction of electrocatalysts and active sites.
|
Sample |
Main Product |
Carbon Precursor |
N Precursor |
Type a |
Synthesis Method |
Nitrogen Species (% atomic) XPS |
Active site |
Ref |
||||||
|
N b |
Pyri c |
Pyrr d |
G e |
O f |
||||||||||
|
NR/CS-900 |
CO |
CS (porous carbon nanosheets) |
Polymerized Aniline with Ammonium Persistence to Polyaniline (Solid) |
Post |
Activation (ZnCl2) and pyrolysis |
5.30 |
1.45 |
1.05 |
2 |
0.8 |
Pyridinic |
[31] |
||
|
NCNTs |
CO |
CNTs |
Acetonitrile- dicyandiamide |
In situ |
Liquid vapor deposition (CVD) |
5.0 |
1.5 |
1.1 |
2.4 |
n.d. |
Pyridinic |
[32] |
||
|
WNCNs-1000 |
CO |
Coal, NaCl template method C-700 |
NH3 |
Post |
Pyrolysis |
4.3 |
2.61 |
1.45 |
0.16 |
0.08 |
Pyridinic |
[33] |
||
|
NCNTs-ACN-850 |
CO |
CNTs (Acetonitrile) |
Dicyandiamide |
In situ |
Liquid chemical vapor deposition |
4.9 |
1 |
0.5 |
3.4 |
n.d. |
Pyridinic |
[21] |
||
|
CN/MWCNT |
CO |
MWCNT |
NaN3 reacts with C3N2Cl3 to form g-C3N4 |
Post |
Pyrolysis |
0.12 |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
[34] |
||
|
NCNT-3-700 |
CO |
CNTs |
Poly(diallyldimethylammonium chloride) (PDDA) |
Post |
Pyrolysis |
1.75 |
0.5 |
0.625 |
0.5 |
0.125 |
Graphitic |
[35] |
||
|
CNPC-1100 |
CO |
Coal |
NH3 |
Post |
Ammonia etching/pyrolysis |
4 |
1 |
1 |
1.5 |
0.5 |
n.d. |
[36] |
||
|
NG-800 |
CO |
GF (Graphene foam) Ni-foam vapor deposition |
gC3N4 (Solid) |
Post |
Pyrolysis |
6.6 |
4.5 |
1.5 |
0.6 |
n.d. |
Pyridinic |
[37] |
||
|
MNC-D |
CO |
ZIF-8 |
Dimethyformamide DMF |
Post |
Heat-treated |
16.97 |
8 |
5 |
4 |
n.d. |
Pyridinic |
[38] |
||
|
NPC-900 |
CO |
Coal arantracite |
Dicyandiamide (DICY) |
In situ |
Activation (KOH)/Pyrolysis |
1.92 |
0.60 |
0.50 |
0.83 |
n.d. |
n.d. |
[27] |
||
|
N-CWM |
CO |
Natural wood |
Urea |
Post |
Pyrolysis |
4.07 |
0.54 |
2.90 |
0.55 |
0.09 |
Pyridinic |
[39] |
||
|
N-graphene |
Formate |
Graphene oxide |
Melamine (Solid) |
In situ |
Pyrolysis |
5.5 |
3 |
0.9 |
1.6 |
n.d. |
Pyridinic |
[40] |
||
|
PEI-NCNT/GC |
Formate |
Multiwalled CNT |
Ammonia plasma/co-catalyst (PEI) |
Post |
Ammonia plasma enhanced chemical vapor deposition (PECVD) |
11.3 |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
[41] |
||
|
HNCM/CNT |
Formate |
CNT |
Dimethyformamide DMF |
In situ |
Pyrolysis |
8.26 |
3.5 |
n.d. |
4.8 |
n.d. |
Pyridinic |
[42] |
||
|
N-C61-800 |
Formate |
Fullerene PC61BM |
Urea |
In situ |
Pyrolysis |
2.57 |
0.39 |
0.54 |
1.64 |
|
Graphitic |
[43] |
||
|
CF-120 |
Syngas |
CF (carbon foam)PU |
NH3 |
Post |
Ammonia etching/pyrolysis |
5.5 |
2.5 |
1.8 |
0.6 |
0.6 |
Pyridinic/Pyrrolic |
[44] |
||
|
3D N-CNTs/SS-750 |
Syngas |
Melamine |
Melamine |
In situ |
Pyrolysis |
6.8 |
3.90 |
0.50 |
2.50 |
n.d. |
n.d. |
[45] |
||
|
c-NC |
Ethanol |
Resol |
Dicyandiamide |
In situ |
Pyrolysis/Template method-mesoporous materials |
7 |
2.6 |
2.8 |
1.6 |
n.d. |
Pyridinic/Pyrrolic |
[46] |
||
|
MNCs-5 |
Ethanol |
Resol |
Dicyandiamide |
In situ |
Pyrolysis/triblock-copolymer-templating method. |
~6.2 |
2.1 |
2.8 |
1.3 |
n.d. |
Pyridinic/Pyrrolic |
[47] |
||
|
GO-VB6-4 |
Ethanol-Acetone |
Graphene oxide |
B6 Vitamin |
Post |
heat treatment |
2.3 |
2.3 |
n.d. |
n.d. |
n.d. |
Pyridinic |
[48] |
||
|
NGM-1/CP |
CH4 |
Graphene |
3-Pyridinecarbonitrile |
In situ |
Pyrolysis |
6.52 |
1.45 |
3.35 |
1.72 |
n.d. |
Pyridinic/Pyrrolic |
[49] |
||
|
Sample |
Main Product |
Carbon Precursor |
N Precursor |
Type a |
Synthesis Method |
Nitrogen Species (% atomic) XPS |
Active site |
Ref |
||||||
|
N b |
Pyri c |
Pyrr d |
G e |
O f |
||||||||||
|
NR/CS-900 |
CO |
CS (porous carbon nanosheets) |
Polymerized Aniline with Ammonium Persistence to Polyaniline (Solid) |
Post |
Activation (ZnCl2) and pyrolysis |
5.30 |
1.45 |
1.05 |
2 |
0.8 |
Pyridinic |
[31] |
||
|
NCNTs |
CO |
CNTs |
Acetonitrile- dicyandiamide |
In situ |
Liquid vapor deposition (CVD) |
5.0 |
1.5 |
1.1 |
2.4 |
n.d. |
Pyridinic |
[32] |
||
|
WNCNs-1000 |
CO |
Coal, NaCl template method C-700 |
NH3 |
Post |
Pyrolysis |
4.3 |
2.61 |
1.45 |
0.16 |
0.08 |
Pyridinic |
[33] |
||
|
NCNTs-ACN-850 |
CO |
CNTs (Acetonitrile) |
Dicyandiamide |
In situ |
Liquid chemical vapor deposition |
4.9 |
1 |
0.5 |
3.4 |
n.d. |
Pyridinic |
[21] |
||
|
CN/MWCNT |
CO |
MWCNT |
NaN3 reacts with C3N2Cl3 to form g-C3N4 |
Post |
Pyrolysis |
0.12 |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
[34] |
||
|
NCNT-3-700 |
CO |
CNTs |
Poly(diallyldimethylammonium chloride) (PDDA) |
Post |
Pyrolysis |
1.75 |
0.5 |
0.625 |
0.5 |
0.125 |
Graphitic |
[35] |
||
|
CNPC-1100 |
CO |
Coal |
NH3 |
Post |
Ammonia etching/pyrolysis |
4 |
1 |
1 |
1.5 |
0.5 |
n.d. |
[36] |
||
|
NG-800 |
CO |
GF (Graphene foam) Ni-foam vapor deposition |
gC3N4 (Solid) |
Post |
Pyrolysis |
6.6 |
4.5 |
1.5 |
0.6 |
n.d. |
Pyridinic |
[37] |
||
|
MNC-D |
CO |
ZIF-8 |
Dimethyformamide DMF |
Post |
Heat-treated |
16.97 |
8 |
5 |
4 |
n.d. |
Pyridinic |
[38] |
||
|
NPC-900 |
CO |
Coal arantracite |
Dicyandiamide (DICY) |
In situ |
Activation (KOH)/Pyrolysis |
1.92 |
0.60 |
0.50 |
0.83 |
n.d. |
n.d. |
[27] |
||
|
N-CWM |
CO |
Natural wood |
Urea |
Post |
Pyrolysis |
4.07 |
0.54 |
2.90 |
0.55 |
0.09 |
Pyridinic |
[39] |
||
|
N-graphene |
Formate |
Graphene oxide |
Melamine (Solid) |
In situ |
Pyrolysis |
5.5 |
3 |
0.9 |
1.6 |
n.d. |
Pyridinic |
[40] |
||
|
PEI-NCNT/GC |
Formate |
Multiwalled CNT |
Ammonia plasma/co-catalyst (PEI) |
Post |
Ammonia plasma enhanced chemical vapor deposition (PECVD) |
11.3 |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
[41] |
||
|
HNCM/CNT |
Formate |
CNT |
Dimethyformamide DMF |
In situ |
Pyrolysis |
8.26 |
3.5 |
n.d. |
4.8 |
n.d. |
Pyridinic |
[42] |
||
|
N-C61-800 |
Formate |
Fullerene PC61BM |
Urea |
In situ |
Pyrolysis |
2.57 |
0.39 |
0.54 |
1.64 |
|
Graphitic |
[43] |
||
|
CF-120 |
Syngas |
CF (carbon foam)PU |
NH3 |
Post |
Ammonia etching/pyrolysis |
5.5 |
2.5 |
1.8 |
0.6 |
0.6 |
Pyridinic/Pyrrolic |
[44] |
||
|
3D N-CNTs/SS-750 |
Syngas |
Melamine |
Melamine |
In situ |
Pyrolysis |
6.8 |
3.90 |
0.50 |
2.50 |
n.d. |
n.d. |
[45] |
||
|
c-NC |
Ethanol |
Resol |
Dicyandiamide |
In situ |
Pyrolysis/Template method-mesoporous materials |
7 |
2.6 |
2.8 |
1.6 |
n.d. |
Pyridinic/Pyrrolic |
[46] |
||
|
MNCs-5 |
Ethanol |
Resol |
Dicyandiamide |
In situ |
Pyrolysis/triblock-copolymer-templating method. |
~6.2 |
2.1 |
2.8 |
1.3 |
n.d. |
Pyridinic/Pyrrolic |
[47] |
||
|
GO-VB6-4 |
Ethanol-Acetone |
Graphene oxide |
B6 Vitamin |
Post |
heat treatment |
2.3 |
2.3 |
n.d. |
n.d. |
n.d. |
Pyridinic |
[48] |
||
|
NGM-1/CP |
CH4 |
Graphene |
3-Pyridinecarbonitrile |
In situ |
Pyrolysis |
6.52 |
1.45 |
3.35 |
1.72 |
n.d. |
Pyridinic/Pyrrolic |
[49] |
||
a N-doping type, b N total, c Pyridinic, d Pyrrolic, e Graphitic, f Oxide.
References
- Kaneco, S.; Kurimoto, H.; Shimizu, Y.; Ohta, K.; Mizuno, T. Photocatalytic reduction of CO2 using TiO2 powders in super-critical fluid CO2. Energy 1999, 24, 21–30, doi:10.1016/S0360-5442(98)00070-X.
- Mori, K.; Yamashita, H.; Anpo, M. Photocatalytic reduction of CO2 with H2O on various titanium oxide photocatalysts. RSC Adv. 2012, 2, 3165–3172, doi:10.1039/C2RA01332K.
- Xie, S.; Zhang, Q.; Liu, G.; Wang, Y. Photocatalytic and photoelectrocatalytic reduction of CO2 using heterogeneous catalysts with controlled nanostructures. Chem. Commun. 2016, 52, 35–59, doi:10.1039/C5CC07613G.
- Wang, C.; Sun, Z.; Zheng, Y.; Hu, Y.H. Recent progress in visible light photocatalytic conversion of carbon dioxide. J. Mater. Chem. A 2019, 7, 865–887, doi:10.1039/C8TA09865D.
- Chueh, W.C.; Haile, S.M. Ceria as a Thermochemical Reaction Medium for Selectively Generating Syngas or Methane from H2O and CO2. ChemSusChem 2009, 2, 735–739, doi:10.1002/cssc.200900138.
- Frey, M.; Édouard, D.; Roger, A.-C. Optimization of structured cellular foam-based catalysts for low-temperature carbon dioxide methanation in a platelet milli-reactor. C. R. Chim. 2015, 18, 283–292, doi:10.1016/j.crci.2015.01.002.
- Roy, S.; Cherevotan, A.; Peter, S.C. Thermochemical CO2 Hydrogenation to Single Carbon Products: Scientific and Techno-logical Challenges. ACS Energy Lett. 2018, 3, 1938–1966, doi:10.1021/acsenergylett.8b00740.
- Ali, N.; Bilal, M.; Nazir, M.S.; Khan, A.; Ali, F.; Iqbal, H.M.N. Thermochemical and electrochemical aspects of carbon dioxide methanation: A sustainable approach to generate fuel via waste to energy theme. Sci. Total Environ. 2020, 712, 136482, doi:10.1016/j.scitotenv.2019.136482.
- Grodkowski, J.; Neta, P. Copper-Catalyzed Radiolytic Reduction of CO2 to CO in Aqueous Solutions. J. Phys. Chem. B 2001, 105, 4967–4972, doi:10.1021/jp004567d.
- Grodkowski, J.; Neta, P. Copper-Catalyzed Radiolytic Reduction of CO2 to CO in Aqueous Solutions. J. Phys. Chem. B 2001, 105, 4967–4972, doi:10.1021/jp004567d.
- Ramirez-Corredores, M.M.; Gadikota, G.; Huang, E.E.; Gaffney, A.M. Radiation-Induced Chemistry of Carbon Dioxide: A Pathway to Close the Carbon Loop for a Circular Economy. Front. Energy Res. 2020, 8, 108, doi:10.3389/fenrg.2020.00108.
- Machado, I.M.P.; Atsumi, S. Cyanobacterial biofuel production. J. Biotechnol. 2012, 162, 50–56, doi:10.1016/j.jbiotec.2012.03.005.
- Rabinovitch-Deere, C.A.; Oliver, J.W.K.; Rodriguez, G.M.; Atsumi, S. Synthetic Biology and Metabolic Engineering Ap-proaches To Produce Biofuels. Chem. Rev. 2013, 113, 4611–4632, doi:10.1021/cr300361t
- Rittmann, S.; Seifert, A.; Herwig, C. Essential prerequisites for successful bioprocess development of biological CH4 pro-duction from CO2 and H2. Crit. Rev. Biotechnol. 2015, 35, 141–151, doi:10.3109/07388551.2013.820685.
- Scibioh, M.A.; Viswanathan, B. Chapter 6—Biochemical Reduction of CO2. In Carbon Dioxide to Chemicals and Fuels; Scibioh, M.A., Viswanathan, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 255–306, ISBN 978-0-444-63996-7.
- Tuci, G.; Filippi, J.; Rossin, A.; Luconi, L.; Pham-Huu, C.; Yakhvarov, D.; Vizza, F.; Giambastiani, G. CO2 electrochemical reduction by exohedral N-pyridine decorated metal-free carbon nanotubes. Energies 2020, 13, 2703, doi:10.3390/en13112703.
- Jia, C.; Dastafkan, K.; Ren, W.; Yang, W.; Zhao, C. Carbon-based catalysts for electrochemical CO2 reduction. Sustain. Energy Fuels 2019, 3, 2890–2906, doi:10.1039/c9se00527g.
- Irabien, A.; Alvarez-Guerra, M.; Albo, J.; Dominguez-Ramos, A. Electrochemical Conversion of CO2 to Value-Added Products; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128131602.
- Mondal, B.; Song, J.; Neese, F.; Ye, S. Bio-inspired mechanistic insights into CO2 reduction. Curr. Opin. Chem. Biol. 2015, 25, 103–109, doi:10.1016/j.cbpa.2014.12.022.
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81, doi:10.1039/B912904A.
- Sharma, P.P.; Wu, J.; Yadav, R.M.; Liu, M.; Wright, C.J.; Tiwary, C.S.; Yakobson, B.I.; Lou, J.; Ajayan, P.M.; Zhou, X.-D. Ni-trogen-Doped Carbon Nanotube Arrays for High-Efficiency Electrochemical Reduction of CO2: On the Understanding of Defects, Defect Density, and Selectivity. Angew. Chem. 2015, 127, 13905–13909, doi:10.1002/ange.201506062
- Fernandes, D.M.; Peixoto, A.F.; Freire, C. Nitrogen-doped metal-free carbon catalysts for (electro)chemical CO2 conversion and valorisation. Dalt. Trans. 2019, 48, 13508–13528, doi:10.1039/c9dt01691k.
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Carbocatalysis by Graphene-Based Materials. Chem. Rev. 2014, 114, 6179–6212, doi:10.1021/cr4007347.
- Ji, Y.; Shi, Y.; Liu, C.; Zhang, B. Plasma-regulated N-doped carbon nanotube arrays for efficient electrosynthesis of syngas with a wide CO/H2 ratio. Sci. China Mater. 2020, 63, 1–7, doi:10.1007/s40843-020-1396-7.
- Hu, C.; Dai, L. Doping of Carbon Materials for Metal-Free Electrocatalysis. Adv. Mater. 2019, 31, 1804672, doi:10.1002/adma.201804672.
- Duan, X.; Xu, J.; Wei, Z.; Ma, J.; Guo, S.; Wang, S.; Liu, H.; Dou, S. Metal-Free Carbon Materials for CO2 Electrochemical Reduction. Adv. Mater. 2017, 29, 1701784, doi:10.1002/adma.201701784.
- Liu, W.; Qi, J.; Bai, P.; Zhang, W.; Xu, L. Utilizing spatial confinement effect of N atoms in micropores of coal-based metal-free material for efficiently electrochemical reduction of carbon dioxide. Appl. Catal. B Environ. 2020, 272, 118974, doi:10.1016/j.apcatb.2020.118974.
- Gao, K.; Wang, B.; Tao, L.; Cunning, B.V.; Zhang, Z.; Wang, S.; Ruoff, R.S.; Qu, L. Efficient Metal-Free Electrocatalysts from N-Doped Carbon Nanomaterials: Mono-Doping and Co-Doping. Adv. Mater. 2019, 31, 1–11, doi:10.1002/adma.201805121.
- Liu, D.; Tao, L.; Yan, D.; Zou, Y.; Wang, S. Recent Advances on Non-precious Metal Porous Carbon-based Electrocatalysts for Oxygen Reduction Reaction. ChemElectroChem 2018, 5, 1775–1785, doi:10.1002/celc.201800086.
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon N. Y. 2018, 132, 104–140, doi:10.1016/j.carbon.2018.02.024.
- Zhu, Y.; Lv, K.; Wang, X.; Yang, H.; Xiao, G.; Zhu, Y. 1D/2D nitrogen-doped carbon nanorod arrays/ultrathin carbon nanosheets: Outstanding catalysts for the highly efficient electroreduction of CO2 to CO. J. Mater. Chem. A 2019, 7, 14895–14903, doi:10.1039/c9ta02353d.
- Wu, J.; Yadav, R.M.; Liu, M.; Sharma, P.P.; Tiwary, C.S.; Ma, L.; Zou, X.; Zhou, X.; Yakobson, B.I.; Lou, J.; et al. Achieving Highly Efficient, Selective, and Stable CO2 Reduction on Nitrogen-Doped Carbon Nanotubes. ACS Nano 2015, 9, 5364–5371.
- Li, H.; Xiao, N.; Hao, M.; Song, X.; Wang, Y.; Ji, Y.; Liu, C.; Li, C.; Guo, Z.; Zhang, F.; et al. Efficient CO2 electroreduction over pyridinic-N active sites highly exposed on wrinkled porous carbon nanosheets. Chem. Eng. J. 2018, 351, 613–621, doi:10.1016/j.cej.2018.06.077.
- Jhong, H.R.M.; Tornow, C.E.; Smid, B.; Gewirth, A.A.; Lyth, S.M.; Kenis, P.J.A. A Nitrogen-Doped Carbon Catalyst for Elec-trochemical CO2 Conversion to CO with High Selectivity and Current Density. ChemSusChem 2017, 10, 1094–1099, doi:10.1002/cssc.201600843.
- Xu, J.; Kan, Y.; Huang, R.; Zhang, B.; Wang, B.; Wu, K.H.; Lin, Y.; Sun, X.; Li, Q.; Centi, G.; et al. Revealing the Origin of Activity in Nitrogen-Doped Nanocarbons towards Electrocatalytic Reduction of Carbon Dioxide. ChemSusChem 2016, 9, 1085–1089, doi:10.1002/cssc.201600202.
- Li, C.; Wang, Y.; Xiao, N.; Li, H.; Ji, Y.; Guo, Z.; Liu, C.; Qiu, J. Nitrogen-doped porous carbon from coal for high efficiency CO2 electrocatalytic reduction. Carbon N. Y. 2019, 151, 46–52, doi:10.1016/j.carbon.2019.05.042.
- Wu, J.; Liu, M.; Sharma, P.P.; Yadav, R.M.; Ma, L.; Yang, Y.; Zou, X.; Zhou, X.D.; Vajtai, R.; Yakobson, B.I.; et al. Incorporation of Nitrogen Defects for Efficient Reduction of CO2 via Two-Electron Pathway on Three-Dimensional Graphene Foam. Nano Lett. 2016, 16, 466–470, doi:10.1021/acs.nanolett.5b04123.
- Kuang, M.; Guan, A.; Gu, Z.; Han, P.; Qian, L.; Zheng, G. Enhanced N-doping in mesoporous carbon for efficient electrocat-alytic CO2 conversion. Nano Res. 2019, 12, 2324–2329, doi:10.1007/s12274-019-2396-6.
- Zhang, H.; Min, S.; Wang, F.; Zhang, Z.; Kong, C. Efficient electrocatalytic CO2 reduction to CO with high selectivity using a N-doped carbonized wood membrane. New J. Chem. 2020, 44, 6125–6129, doi:10.1039/d0nj00538j.
- Wang, H.; Chen, Y.; Hou, X.; Ma, C.; Tan, T. Nitrogen-doped graphenes as efficient electrocatalysts for the selective reduction of carbon dioxide to formate in aqueous solution. Green Chem. 2016, 18, 3250–3256, doi:10.1039/c6gc00410e.
- Zhang, S.; Kang, P.; Ubnoske, S.; Brennaman, M.K.; Song, N.; House, R.L.; Glass, J.T.; Meyer, T.J. Polyethylenimine-enhanced electrocatalytic reduction of CO2 to formate at nitrogen-doped carbon nanomaterials. J. Am. Chem. Soc. 2014, 136, 7845–7848, doi:10.1021/ja5031529.
- Wang, H.; Jia, J.; Song, P.; Wang, Q.; Li, D.; Min, S.; Qian, C.; Wang, L.; Li, Y.F.; Ma, C.; et al. Efficient Electrocatalytic Reduction of CO2 by Nitrogen-Doped Nanoporous Carbon/Carbon Nanotube Membranes: A Step Towards the Electrochemical CO2 Refinery. Angew. Chem. Int. Ed. 2017, 56, 7847–7852, doi:10.1002/anie.201703720.
- Chen, Z.; Mou, K.; Yao, S.; Liu, L. Highly selective electrochemical reduction of CO2 to formate on metal-free nitrogen-doped PC61BM. J. Mater. Chem. A 2018, 6, 11236–11243, doi:10.1039/c8ta03328e.
- Li, H.; Xiao, N.; Wang, Y.; Li, C.; Ye, X.; Guo, Z.; Pan, X.; Liu, C.; Bai, J.; Xiao, J.; et al. Nitrogen-doped tubular carbon foam electrodes for efficient electroreduction of CO2 to syngas with potential-independent CO/H2 ratios. J. Mater. Chem. A 2019, 7, 18852–18860, doi:10.1039/c9ta05904k.
- Liu, K.H.; Zhong, H.X.; Yang, X.Y.; Bao, D.; Meng, F.L.; Yan, J.M.; Zhang, X.B. Composition-tunable synthesis of “clean” syngas: Via a one-step synthesis of metal-free pyridinic-N-enriched self-supported CNTs: The synergy of electrocatalyst py-rolysis temperature and potential. Green Chem. 2017, 19, 4284–4288, doi:10.1039/c7gc01095h.
- Song, Y.; Chen, W.; Zhao, C.; Li, S.; Wei, W.; Sun, Y. Metal-Free Nitrogen-Doped Mesoporous Carbon for Electroreduction of CO2 to Ethanol. Angew. Chem. Int. Ed. 2017, 56, 10840–10844, doi:10.1002/anie.201706777.
- Song, Y.; Wang, S.; Chen, W.; Li, S.; Feng, G.; Wei, W.; Sun, Y. Enhanced Ethanol Production from CO2 Electroreduction at Micropores in Nitrogen-Doped Mesoporous Carbon. ChemSusChem 2020, 13, 293–297, doi:10.1002/cssc.201902833.
- Yuan, J.; Zhi, W.Y.; Liu, L.; Yang, M.P.; Wang, H.; Lu, J.X. Electrochemical reduction of CO2 at metal-free N-functionalized graphene oxide electrodes. Electrochim. Acta 2018, 282, 694–701, doi:10.1016/j.electacta.2018.06.107.
- Sun, X.; Kang, X.; Zhu, Q.; Ma, J.; Yang, G.; Liu, Z.; Han, B. Very highly efficient reduction of CO2 to CH4 using metal-free N-doped carbon electrodes. Chem. Sci. 2016, 7, 2883–2887, doi:10.1039/C5SC04158A.