The plant circadian clock has a pervasive influence on many aspects of plant biology and is proposed to function as a developmental manager. To do so, the circadian oscillator needs to be able to integrate a multiplicity of environmental signals and coordinate an extensive and diverse repertoire of endogenous rhythms accordingly. Recent studies on tissue-specific characteristics and spatial structure of the plant circadian clock suggest that such plasticity may be achieved through the function of distinct oscillators, which sense the environment locally and are then coordinated across the plant through both intercellular coupling and long-distance communication.
- circadian clock
- plant
- tissue-specific
- coupling
- spatial organization
- organismal synchronization
1. Introduction
A circadian clock is an endogenous molecular mechanism that generates 24 h rhythms in a wide array of biological processes. As a consequence of the Earth’s rotation, organisms have evolved these timing mechanisms to align their physiology and development with the periodic changes in environmental conditions that occur over the day-night cycle. In the natural environment, the ability to track time enables organisms to anticipate these conditions and adequately coordinate different processes to occur at the most appropriate times. The anticipatory behavior conferred by these biological oscillators thus allows for an efficient distribution and use of metabolic resources and is thought to provide an adaptive advantage [1]. In fact, plant circadian mutants with dysfunctional clocks display reduced photosynthesis, growth and viability, especially under challenging conditions [1][2][3]. While the internal circadian oscillator runs with an intrinsic free-running period of approximately 24 h, it is synchronized, or entrained, to the exact period of environmental cycles through its sensitivity to multiple input signals, both exogenous and endogenous [4]. Light and temperature play a major role in the entrainment of the plant clock [5], which is also affected by other factors including humidity [6], ions [7][8] and metabolites [9][10].
A circadian clock is an endogenous molecular mechanism that generates 24 h rhythms in a wide array of biological processes. As a consequence of the Earth’s rotation, organisms have evolved these timing mechanisms to align their physiology and development with the periodic changes in environmental conditions that occur over the day-night cycle. In the natural environment, the ability to track time enables organisms to anticipate these conditions and adequately coordinate different processes to occur at the most appropriate times. The anticipatory behavior conferred by these biological oscillators thus allows for an efficient distribution and use of metabolic resources and is thought to provide an adaptive advantage [1]. In fact, plant circadian mutants with dysfunctional clocks display reduced photosynthesis, growth and viability, especially under challenging conditions [1,2,3]. While the internal circadian oscillator runs with an intrinsic free-running period of approximately 24 h, it is synchronized, or entrained, to the exact period of environmental cycles through its sensitivity to multiple input signals, both exogenous and endogenous [4]. Light and temperature play a major role in the entrainment of the plant clock [5], which is also affected by other factors including humidity [6], ions [7,8] and metabolites [9,10].
Almost every aspect of plant physiology and development is subject to some extent of circadian regulation and many efforts have been devoted towards the identification of the genes and proteins that constitute the core molecular mechanism driving this pervasive rhythmicity. As a result, multiple clock components and intricate reciprocal regulatory connections have been identified [5][11][12] (
Almost every aspect of plant physiology and development is subject to some extent of circadian regulation and many efforts have been devoted towards the identification of the genes and proteins that constitute the core molecular mechanism driving this pervasive rhythmicity. As a result, multiple clock components and intricate reciprocal regulatory connections have been identified [5,11,12] (
Figure 1). Similarly to other organisms [13], the central oscillator in plants is composed of numerous transcriptional-translational loops where clock genes exert feedback regulation on each other, providing this timing mechanism and ultimately driving rhythmic expression of a significant portion of the transcriptome [14][15]. Two single MYB-domain transcription factors, CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATEDHYPOCOTYL (LHY), are expressed at dawn and they directly repress the expression of morning- and evening-phased clock genes, as well as their own expression [16][17]. As the day progresses, members of the PSEUDO-RESPONSE REGULATOR (PRR) family (PRR9, PRR7, PRR5 and PRR1 (better known as TIMING OF CAB EXPRESSION 1, TOC1)) are sequentially expressed and they repress
). Similarly to other organisms [13], the central oscillator in plants is composed of numerous transcriptional-translational loops where clock genes exert feedback regulation on each other, providing this timing mechanism and ultimately driving rhythmic expression of a significant portion of the transcriptome [14,15]. Two single MYB-domain transcription factors, CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATEDHYPOCOTYL (LHY), are expressed at dawn and they directly repress the expression of morning- and evening-phased clock genes, as well as their own expression [16,17]. As the day progresses, members of the PSEUDO-RESPONSE REGULATOR (PRR) family (PRR9, PRR7, PRR5 and PRR1 (better known as TIMING OF CAB EXPRESSION 1, TOC1)) are sequentially expressed and they repress
CCA1
and
LHY, as well as each other [18][19][20]. In the evening, TOC1 represses all the previously expressed and aforementioned clock components, including
, as well as each other [18,19,20]. In the evening, TOC1 represses all the previously expressed and aforementioned clock components, including
GIGANTEA
(
GI
),
LUX ARRYTHMO
(
LUX
) and
EARLY FLOWERING 4
(
ELF4
) [20]. Later during the night, a tripartite complex conformed by ELF3, ELF4 and LUX (the Evening Complex, EC) maintains the repression of
GI
and represses
PRR9
,
PRR7
and
LUX
and likely indirectly induces
CCA1
and
LHY
expression [21].
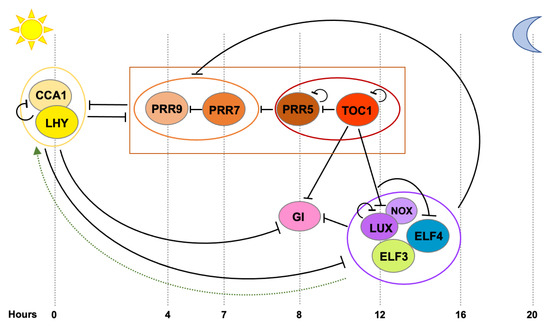
Figure 1.
Arabidopsis thaliana
PRRs
GI
LUX
ELF3
ELF4
CCA1
LHY
CCA1
LHY
PRRs
GI
LUX
ELF4
GI
PRR9
PRR7
CCA1
LHY.
2. Tissue-Specificity of the Plant Circadian Clock
2.1. Early Evidences for Tissue-Specific Clocks
The existence of multiple oscillators in plants has long been proposed. Early studies in bean plants evidenced that the free-running period of stomatal opening and photosynthesis was different to that of leaflet movement [22] and studies in tobacco plants showed that rhythms in cytosolic free calcium (Ca
The existence of multiple oscillators in plants has long been proposed. Early studies in bean plants evidenced that the free-running period of stomatal opening and photosynthesis was different to that of leaflet movement [24] and studies in tobacco plants showed that rhythms in cytosolic free calcium (Ca
2+
) levels also free-run with a different period than the expression of a light-harvesting complex (
Lhc
)
b gene family member [23]. A single clock can control multiple rhythms with different phases, but it will only render one period, as period is an intrinsic property of the oscillator. Hence, it was deduced that the difference in free-running periods displayed by these rhythms arose from the function of multiple separate plant oscillators with different intrinsic frequencies. Tissue-specific properties of these pacemakers were further investigated by analyzing a single rhythm, namely cytosolic free Ca
gene family member [25]. A single clock can control multiple rhythms with different phases, but it will only render one period, as period is an intrinsic property of the oscillator. Hence, it was deduced that the difference in free-running periods displayed by these rhythms arose from the function of multiple separate plant oscillators with different intrinsic frequencies. Tissue-specific properties of these pacemakers were further investigated by analyzing a single rhythm, namely cytosolic free Ca
2+ oscillations, in different tissues [24]. For this, transgenic tobacco plants expressing the aequorin protein (a luminescent reporter for Ca
oscillations, in different tissues [26]. For this, transgenic tobacco plants expressing the aequorin protein (a luminescent reporter for Ca
2+
levels) driven by different promoters with distinct spatial patterns of expression were generated. Under free-running conditions, circadian oscillations in Ca
2+
exhibited distinct phases in each line. While these findings do not necessarily imply the function of separate oscillators, they evidence the existence of distinct cellular control mechanisms contributing to circadian rhythms in Ca
2+ levels [24].
levels [26].
To inspect whether the different oscillators were composed of similar components or not, the effect of mutations in the central oscillator and light input pathway genes on presumably independent rhythms was analyzed. These rhythms comprised oscillations in cytosolic Ca
2+
levels, as well as in
CHALCONE SYNTHASE
(
CHS
),
CHLOROPHYLL A/B BINDING PROTEIN
(
CAB
) and
PHYTOCHROME B
(
PHYB) promoter activity, which owing to their distinct spatial distribution patterns and free-running periods were suggested to be regulated by separate oscillators located in different cell types [25][26][27]. Genetic analyses revealed that two clock-affecting mutations (in the core clock gene
) promoter activity, which owing to their distinct spatial distribution patterns and free-running periods were suggested to be regulated by separate oscillators located in different cell types [27,28,29]. Genetic analyses revealed that two clock-affecting mutations (in the core clock gene
TOC1
and the light signaling component
DE-ETIOLATED 1
,
DET1
) similarly affected the period of
CHS
and
CAB promoter activity [25]. Likewise, misexpression of the red-light photoreceptor
promoter activity [27]. Likewise, misexpression of the red-light photoreceptor
PHYB
and the core clock genes
CCA1
,
LHY
and
ELF3
also affected the period of both
CAB
and
PHYB promoter activity in a similar fashion, hence suggesting that the separate oscillators share common components [26]. Further supporting this notion, it was observed that rhythms in cytosolic Ca
promoter activity in a similar fashion, hence suggesting that the separate oscillators share common components [28]. Further supporting this notion, it was observed that rhythms in cytosolic Ca
2+
levels and
CAB
promoter activity were both dependent on
CCA1
,
LHY
and
TOC1 function [27]. However, this study also revealed that a semidominant allele of
function [29]. However, this study also revealed that a semidominant allele of
TOC1
(
toc1-1
), which contains an amino acid change in the conserved CCT (for CONSTANS, CONSTANS-LIKE and TOC1) domain, uncoupled both rhythms and only affected
CAB oscillations [27]. Thus, these findings indicated that although the separate oscillators do seem to share common components, these may function or relate to each other differently in each tissue to render different frequencies.
oscillations [29]. Thus, these findings indicated that although the separate oscillators do seem to share common components, these may function or relate to each other differently in each tissue to render different frequencies.
2.2. Mechanisms Underlying Tissue-Specific Circadian Rhythms
Local differences in circadian rhythmicity from a similar oscillator can be achieved through various mechanisms including diverging levels in core clock gene expression, functional modulation of these clock genes by tissue-specific regulators and/or through differential perception of input signals.
The majority of clock genes are rhythmically expressed across the entire plant [28][29][30][31], but tissue-specific expression levels and circadian properties have been reported for many of them. Comparison of
The majority of clock genes are rhythmically expressed across the entire plant [30,31,32,33], but tissue-specific expression levels and circadian properties have been reported for many of them. Comparison of
CCA1
promoter activity under free-running conditions in the center of the leaf with that in the center of the rosette of
Arabidopsis thaliana plants revealed differences in period length depending on the organ [32].
plants revealed differences in period length depending on the organ [34].
CCA1 was also reported to display lower expression levels and a longer free-running period in guard cells compared to whole leaves [33]. A similar behavior was also observed for other oscillator components such as
was also reported to display lower expression levels and a longer free-running period in guard cells compared to whole leaves [35]. A similar behavior was also observed for other oscillator components such as
LHY
,
TOC1
and
CCA1 HIKING EXPEDITION
(
CHE) [33]. Interestingly, in the same study it was observed that another clock gene,
) [35]. Interestingly, in the same study it was observed that another clock gene,
GI, also had a later phase and run with a longer period in guard cells, but displayed similar expression levels in this cell type compared to whole leaves [33]. Hence, individual clock components behave differently across the plant. In terms of expression levels,
, also had a later phase and run with a longer period in guard cells, but displayed similar expression levels in this cell type compared to whole leaves [35]. Hence, individual clock components behave differently across the plant. In terms of expression levels,
GI seems to be more highly expressed in the vasculature [34], similarly to
seems to be more highly expressed in the vasculature [36], similarly to
PRR3
, which is suggested to regulate
TOC1 protein stability in this tissue [29]. Further evidence on tissue-specific variations in the expression levels of core clock components was later provided by a genome-wide gene expression analysis in isolated vasculature and mesophyll cells compared to whole leaves [35]. It was observed that morning expressed clock genes such as
protein stability in this tissue [31]. Further evidence on tissue-specific variations in the expression levels of core clock components was later provided by a genome-wide gene expression analysis in isolated vasculature and mesophyll cells compared to whole leaves [37]. It was observed that morning expressed clock genes such as
CCA1
,
LHY
,
PRR9
and
PRR7
are more highly expressed in mesophyll, while expression of evening phased genes such as
TOC1
,
ELF4
and
LUX is higher in the vasculature [35]. Differences in the expression levels of clock genes have also been observed between shoots and roots [36]. Interestingly, morning and evening phased clock components seem to have a varying impact on circadian function in roots compared to shoots and mutation of several such components affects clock function differently in each organ [36][37][38][39][40], which indicates that the clock network might be wired differently in each case. Hence, divergence in the expression levels and tissue-specific molecular connections among core clock components is likely one of the mechanisms through which distinct local rhythms are achieved.
is higher in the vasculature [37]. Differences in the expression levels of clock genes have also been observed between shoots and roots [38]. Interestingly, morning and evening phased clock components seem to have a varying impact on circadian function in roots compared to shoots and mutation of several such components affects clock function differently in each organ [38,39,40,41,42], which indicates that the clock network might be wired differently in each case. Hence, divergence in the expression levels and tissue-specific molecular connections among core clock components is likely one of the mechanisms through which distinct local rhythms are achieved.
Differential processing of environmental signals is another factor that may contribute to tissue-specific circadian regulation. Because different parts of the plant are exposed to different microenvironments, it is anticipated that the impact of specific environmental cues, such as light quality and quantity, temperature or nutrient levels, will differ. Local differences in the clock’s sensitivity to a wide array of signals would enhance plasticity and could allow the clock to better adapt to ambient conditions locally [4][41].
Differential processing of environmental signals is another factor that may contribute to tissue-specific circadian regulation. Because different parts of the plant are exposed to different microenvironments, it is anticipated that the impact of specific environmental cues, such as light quality and quantity, temperature or nutrient levels, will differ. Local differences in the clock’s sensitivity to a wide array of signals would enhance plasticity and could allow the clock to better adapt to ambient conditions locally [4,43].
Perhaps the most important entraining signal in plants is light. Plants use different classes of photoreceptors to sense the light environment and set the clock to the actual pace of day-night cycles [42]. These photoreceptors include PHYs and CRYPTOCHROMEs (CRYs), which transmit red and blue light signals, respectively [42]. Mutations in both PHY and CRY photoreceptors have been shown to affect circadian period length in response to different light qualities [43][44] and the spatial expression pattern of
Perhaps the most important entraining signal in plants is light. Plants use different classes of photoreceptors to sense the light environment and set the clock to the actual pace of day-night cycles [44]. These photoreceptors include PHYs and CRYPTOCHROMEs (CRYs), which transmit red and blue light signals, respectively [44]. Mutations in both PHY and CRY photoreceptors have been shown to affect circadian period length in response to different light qualities [45,46] and the spatial expression pattern of
PHY
and
CRY genes varies among tissues [45][46][47]. Therefore, local differences in the sensitivity to light via these photoreceptors could be part of the mechanism underlying tissue-specific functions of the clock. Recent reports suggest that the differences in period length between shoots and roots can in fact be explained by different light inputs [36][48], in addition to other input signals such as metabolic sugars [48]. While the free-running period in roots and shoots is fairly similar under constant darkness, the period length of the root clock is considerably longer than the one in shoots under constant light conditions. Furthermore, the root clock is slowed down by blue light compared to red light, whereas the shoot clock showed similar periods in both blue and red light, evidencing differences in light perception and/or signal transduction in both organs [36]. Recent data suggest that function of the evening complex may in fact be part of the light input mechanism that differs between roots and shoots [39]. Additionally, tissue-specific functions of PHYB [49][50] and CRY2 [51], as well as other light signaling components that affect light input to the clock, such as COP1 [52], SPA1 [53] and PIFs [54][55][56], have been reported and could therefore contribute to local differences in the response to light. Further investigation will be required to uncover the overall topology of these tissue-specific light input networks and mechanistically define their function in clock rhythmicity.
genes varies among tissues [47,48,49]. Therefore, local differences in the sensitivity to light via these photoreceptors could be part of the mechanism underlying tissue-specific functions of the clock. Recent reports suggest that the differences in period length between shoots and roots can in fact be explained by different light inputs [38,50], in addition to other input signals such as metabolic sugars [50]. While the free-running period in roots and shoots is fairly similar under constant darkness, the period length of the root clock is considerably longer than the one in shoots under constant light conditions. Furthermore, the root clock is slowed down by blue light compared to red light, whereas the shoot clock showed similar periods in both blue and red light, evidencing differences in light perception and/or signal transduction in both organs [38]. Recent data suggest that function of the evening complex may in fact be part of the light input mechanism that differs between roots and shoots [41]. Additionally, tissue-specific functions of PHYB [51,52] and CRY2 [53], as well as other light signaling components that affect light input to the clock, such as COP1 [54], SPA1 [55] and PIFs [56,57,58], have been reported and could therefore contribute to local differences in the response to light. Further investigation will be required to uncover the overall topology of these tissue-specific light input networks and mechanistically define their function in clock rhythmicity.
In addition to light, temperature is another signal that conveys important information about the surrounding environment. Early studies showed that two separate oscillators involved in the regulation of
CAB2
and
CATALASE 3
(
CAT3
) expression had different sensitivity to light and temperature. The pacemaker regulating
CAB2
gene expression seemed to preferentially respond to light–dark cycles, while the one controlling
CAT3 expression, was more sensitive to temperature signals [57]. More recent studies have also reported differential processing of light and temperature signals in different tissues. By analyzing
expression, was more sensitive to temperature signals [59]. More recent studies have also reported differential processing of light and temperature signals in different tissues. By analyzing
TOC1 promoter activity oscillations in the vasculature compared to whole leaves under light-dark and temperature cycles, it was seen that the vascular clock has lower sensitivity to temperature and higher sensitivity to photoperiodic signals [58]. In fact, an oscillator located in vascular phloem companion cells plays an essential role in photoperiodic flowering control [35][59]. Conversely, a clock in the epidermis seems to display a higher sensitivity to ambient temperature and be required to coordinate other output processes such as temperature-dependent cell elongation [59]. Differences in the response to temperature between shoots and roots have also been documented. Temperature seems to have a more prominent effect on clock speed in roots and this is likely dependent on
promoter activity oscillations in the vasculature compared to whole leaves under light-dark and temperature cycles, it was seen that the vascular clock has lower sensitivity to temperature and higher sensitivity to photoperiodic signals [60]. In fact, an oscillator located in vascular phloem companion cells plays an essential role in photoperiodic flowering control [37,61]. Conversely, a clock in the epidermis seems to display a higher sensitivity to ambient temperature and be required to coordinate other output processes such as temperature-dependent cell elongation [61]. Differences in the response to temperature between shoots and roots have also been documented. Temperature seems to have a more prominent effect on clock speed in roots and this is likely dependent on
PRR9
and
Altogether, several pieces of evidence suggest that local differences in circadian function may arise from a combination of factors, including heterogeneity in the expression levels of core clock components, tissue-specific connections within the circadian network and differential sensitivity and processing of environmental input signals.
