Normal-pressure hydrocephalus (NPH) is a neurological disease characterized by enlarged cerebral ventricles and clinical features of gait disturbance, urinary incontinence, and cognitive decline.
- normal-pressure hydrocephalus
- normal-tension glaucoma
1. Introduction
Normal-pressure hydrocephalus (NPH) is a neurological disease characterized by enlarged cerebral ventricles and clinical features of gait disturbance, urinary incontinence, and cognitive decline [1][2][3]. Dilation of ventricles is caused by a disturbance in the cerebrospinal fluid (CSF) pathway from production to absorption locations [4]. As the name of this disease suggests, intracranial pressure (ICP) remains in the normal range most of the time [5]. However, the name of normal-pressure hydrocephalus is misleading because continuous monitoring of ICP shows intermittently raised ICP in association with pressure waves [6]. NPH is generally considered to be a disorder of adult and geriatric patients. Around 5.5 patients per 100,000 population undergo ventriculoperitoneal (VP) shunt placement for the treatment of NPH annually, representing one of the commonly performed neurosurgical interventions [7]. VP shunt placement relieves this life-threatening disorder [8], but a hypothesis has recently emerged that treatment of NPH is associated with the development of normal-tension glaucoma (NTG) [9][10].
Glaucoma is a chronic, multifactorial optic nerve (ON) disease characterized by progressive retinal nerve fibers and visual field decline [11]. NTG is a subset of open-angle glaucoma in which intraocular pressure (IOP) is normal (IOP ≤ 21 mmHg), in contrast to high-tension glaucoma (HTG) in which IOP is above 21 mmHg [11][12].
The purpose of this article is to review the literature published regarding the association between the treatment of NPH and the development of NTG, to emphasize the need for neuro-ophthalmic follow-up for patients with shunt-treated NPH.
2. Pathophysiologic Mechanism of ON Damage in NTG
The pathology of glaucoma may worsen progressively and irreversibly even in cases where IOP, the main risk factor for glaucoma, does not exceed the normal range. Thus, the cause of ON damage in NTG remains a mystery.
It has been suggested that disturbances in ocular blood flow are a major risk factor in the pathogenesis of NTG [13][14]. Vascular complication, such as vasospasms, vasosclerosis, small vessel disease, and autoregulatory dysfunction, leading to perfusion deficits of the ON head, the retina, the choroid or the retrobulbar vessels, might influence the loss of retinal nerve fibers [13][15]. Systemic hypotension, particularly nocturnal arterial hypotension, is another risk factor that is believed to influence the progression of glaucoma [16][17]. Ocular perfusion pressure is defined as the difference between arterial blood pressure (ABP) and IOP. Thus, low ABP causes low ocular perfusion pressure, and this results in ischemic damage to the ON [16]. Yet other pathogenetic factors, such as autoimmunity [18][19], inflammation [20], and accumulation of toxins [21], are believed to contribute to the development of NTG.
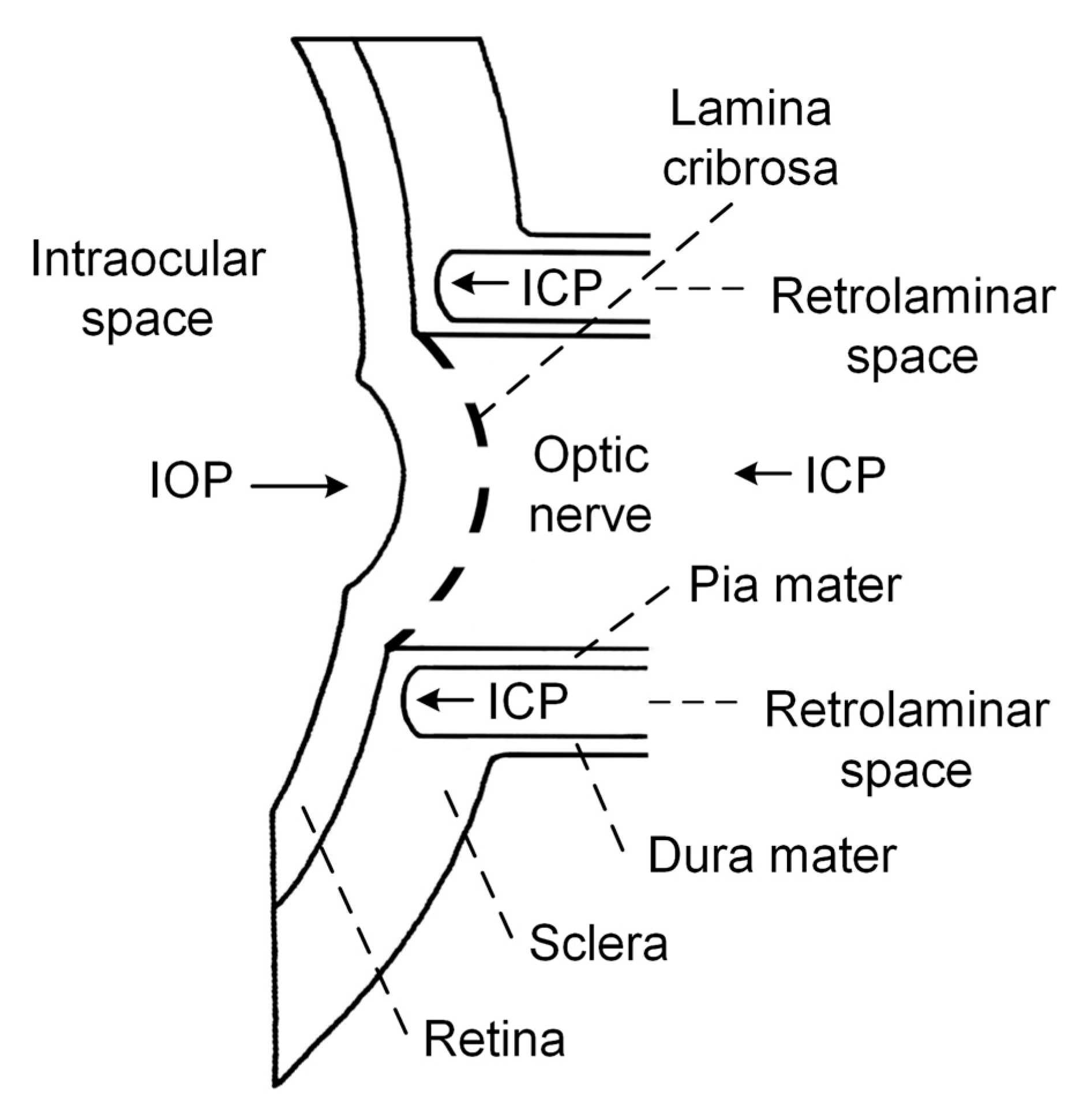
Currently, attention has been focused on the idea formulated by Volkov back in 1976 that a low ICP may be involved in the pathogenesis of glaucomatous optic neuropathy, because the ON immediately beyond the lamina cribrosa (LC) is surrounded by CSF [22]. Schematic representation of the relevant intraocular/retrolaminar space is depicted in Figure 1 for the explanation of this hypothesis.
The LC is a sieve-like structure, through which the retinal ganglion cell axons pass before forming the ON [23]. The retrolaminar space is a CSF-filled compartment surrounding the ON immediately posterior to the LC [24]. The LC separates the intraocular and retrolaminar spaces and is the possible location of axonal injury.
The pressure change across the LC, called translaminar cribrosa pressure gradient (TCPG), has been proposed to be the primary factor responsible for axonal injury [24][25]. TCPG is calculated as TCPG = (IOP-ICP)/thickness of the LC. According to this hypothesis, TCPG increases due to increased IOP or decreased ICP, and could be responsible for ON damage [26]. In the case of NTG, IOP is within a normal range; therefore, decreases in of ICP might be the cause of ON damage.
The first evidence in favor of this hypothesis appeared in 1979, 3 years after the publication by Volkov. In an experimental study, Yablonski et al. slightly decreased the ICP below the atmospheric pressure by cannulating the cisterna magma in cats [27]. The IOP in one eye was decreased to slightly exceed atmospheric pressure by cannulation of the anterior chamber, whereas the remaining eye was left untouched. After 3 weeks, the optic disc of the eye in which IOP was unchanged showed glaucomatous optic neuropathy; in contrast, no sign of glaucoma was identified in the eye that had reduced IOP. Since then, little attention has been focused on the role of ICP in the development of glaucoma until recent years.
In a retrospective study, Berdahl et al. reviewed the medical records of 62,468 patients who had lumbar puncture readings [28]. These authors selected 57 open-angle glaucoma patients (including 11 NTG patients) and 105 age-matched control subjects (patients with no signs of glaucoma) for the comparison of ICP. The mean ICP was significantly lower in open-angle glaucoma patients (9.6 ± 3.1 mmHg) and NTG patients (9.3 ± 3.2 mmHg) compared with control subjects (12.7 ± 3.9 mmHg).
In a prospective study, Ren et al. measured ICP via lumbar puncture on 29 HTG patients, 14 NTG patients and 71 subjects without glaucoma [29]. The mean ICP was significantly lower in NTG patients (9.5 ± 2.2 mmHg) than in the control subjects (12.9 ± 1.9 mmHg) and HTG group (11.7 ± 2.7 mmHg).
In a prospective pilot study, Siaudvytyte and colleagues measured ICP using a novel two-depth transcranial Doppler (TCD) based non-invasive ICP measurement device, including nine patients with NTG, nine patients with HTG, and nine healthy controls [30]. The mean ICP was found to be lower in NTG patients (7.4 ± 2.7 mmHg) than healthy controls (10.5 ± 3.0 mmHg) and the HTG group (8.9 ± 1.9 mmHg), although differences between the groups were not statistically significant.
In a most recent prospective study, conducted by Linden and colleagues, ICP was measured via lumbar puncture by using the Likvor CELDA system on 13 NTG patients and 51 healthy volunteers [31]. The mean ICP measured in a supine body position was lower in the NTG patients (10.3 ± 2.7 mmHg) than healthy volunteers (11.3 ± 2.2 mmHg). However, differences between the groups were not statistically significant.
Although all four studies showed lower mean ICP values measured on NTG patients compared with control subjects, a final conclusion in favor of the proposed hypothesis cannot yet be stated. The largest study was retrospective, and the remaining three, although prospective, did not enroll a large group of NTG patients and could be titled pilot studies.
Dysfunction of an occlusion mechanism of the ON sheath around the ON has been proposed recently as a pathophysiologic component in NTG [32]. The unifying glymphatic hypothesis of glaucoma, which incorporates vascular, biomechanical, and biochemical factors to explain the pathophysiology of glaucomatous optic neuropathy, has also been proposed [25][33].
References
- Kotagal, V.; Walkowiak, E.; Heth, J.A. Serious adverse events following Normal Pressure Hydrocephalus surgery. Clin. Neurol. Neurosurg. 2018, 170, 113–115.
- Lalou, A.D.; Czosnyka, M.; Donnelly, J.; Pickard, J.D.; Nabbanja, E.; Keong, N.C.; Garnett, M.; Czosnyka, Z.H. Cerebral autoregulation, cerebrospinal fluid outflow resistance, and outcome following cerebrospinal fluid diversion in normal pressure hydrocephalus. J. Neurosurg. JNS 2018, 130, 154–162.
- Shprecher, D.; Schwalb, J.; Kurlan, R. Normal pressure hydrocephalus: Diagnosis and treatment. Curr. Neurol. Neurosci. Rep. 2008, 8, 371–376.
- Gholampour, S. FSI simulation of CSF hydrodynamic changes in a large population of non-communicating hydrocephalus patients during treatment process with regard to their clinical symptoms. PLoS ONE 2018, 13, e0196216.
- Czosnyka, Z.; Czosnyka, M. Long-term monitoring of intracranial pressure in normal pressure hydrocephalus and other CSF disorders. Acta Neurochir. (Wien.) 2017, 159, 1979–1980.
- Wostyn, P.; Audenaert, K.; De Deyn, P.P. Alzheimer’s disease-related changes in diseases characterized by elevation of intracranial or intraocular pressure. Clin. Neurol. Neurosurg. 2008, 110, 101–109.
- Agarwal, N.; Kashkoush, A.; McDowell, M.M.; Lariviere, W.R.; Ismail, N.; Friedlander, R.M. Comparative durability and costs analysis of ventricular shunts. J. Neurosurg. JNS 2018, 130, 1252–1259.
- Paulsen, A.H.; Due-Tønnessen, B.J.; Lundar, T.; Lindegaard, K.-F. Cerebrospinal fluid (CSF) shunting and ventriculocisternostomy (ETV) in 400 pediatric patients. Shifts in understanding, diagnostics, case-mix, and surgical management during half a century. Child’s Nerv. Syst. 2017, 33, 259–268.
- Bokhari, R.F.; Baeesa, S.S. Does the treatment of normal pressure hydrocephalus put the retinal ganglion cells at risk? A brief literature review and novel hypothesis. Med. Hypotheses 2013, 81, 686–689.
- Wostyn, P.; Audenaert, K.; De Deyn, P.P. High Occurrence Rate of Glaucoma Among Patients with Normal Pressure Hydrocephalus. J. Glaucoma 2010, 19, 225–226.
- Pircher, A.; Montali, M.; Wostyn, P.; Pircher, J.; Berberat, J.; Remonda, L.; Killer, H.E. Impaired cerebrospinal fluid dynamics along the entire optic nerve in normal-tension glaucoma. Acta Ophthalmol. 2018, 96, e562–e569.
- Xu, H.; Zhai, R.; Zong, Y.; Kong, X.; Jiang, C.; Sun, X.; He, Y.; Li, X. Comparison of retinal microvascular changes in eyes with high-tension glaucoma or normal-tension glaucoma: A quantitative optic coherence tomography angiographic study. Graefe’s Arch. Clin. Exp. Ophthalmol. = Albr. von Graefes Arch. fur Klin. Exp. Ophthalmol. 2018, 256, 1179–1186.
- Plange, N.; Remky, A.; Arend, O. Colour Doppler imaging and fluorescein filling defects of the optic disc in normal tension glaucoma. Br. J. Ophthalmol. 2003, 87, 731–736.
- Sugiyama, T.; Utsunomiya, K.; Ota, H.; Ogura, Y.; Narabayashi, I.; Ikeda, T. Comparative study of cerebral blood flow in patients with normal-tension glaucoma and control subjects. Am. J. Ophthalmol. 2006, 141, 394–396.
- Fan, N.; Wang, P.; Tang, L.; Liu, X. Ocular Blood Flow and Normal Tension Glaucoma. Biomed Res. Int. 2015, 12, 308505.
- Levine, R.M.; Yang, A.; Brahma, V.; Martone, J.F. Management of Blood Pressure in Patients with Glaucoma. Curr. Cardiol. Rep. 2017, 19, 109.
- Hayreh, S.S.; Zimmerman, M.B.; Podhajsky, P.; Alward, W.L. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am. J. Ophthalmol. 1994, 117, 603–624.
- Grus, F.H.; Joachim, S.C.; Wuenschig, D.; Rieck, J.; Pfeiffer, N. Autoimmunity and glaucoma. J. Glaucoma 2008, 17, 79–84.
- Wax, M.B. The case for autoimmunity in glaucoma. Exp. Eye Res. 2011, 93, 187–190.
- Vohra, R.; Tsai, J.C.; Kolko, M. The role of inflammation in the pathogenesis of glaucoma. Surv. Ophthalmol. 2013, 58, 311–320.
- Lee, S.H.; Kang, E.M.; Kim, G.A.; Kwak, S.W.; Kim, J.M.; Bae, H.W.; Seong, G.J.; Kim, C.Y. Three Toxic Heavy Metals in Open-Angle Glaucoma with Low-Teen and High-Teen Intraocular Pressure: A Cross-Sectional Study from South Korea. PLoS ONE 2016, 11, e0164983.
- Volkov, V. V Essential element of the glaucomatous process neglected in clinical practice. Oftalmol. Zh. 1976, 31, 500–504.
- Morgan-Davies, J.; Taylor, N.; Hill, A.R.; Aspinall, P.; O’Brien, C.J.; Azuara-Blanco, A. Three dimensional analysis of the lamina cribrosa in glaucoma. Br. J. Ophthalmol. 2004, 88, 1299–1304.
- McCulley, T.J.; Chang, J.R.; Piluek, W.J. Intracranial pressure and glaucoma. J. Neuro-Ophthalmol. Off. J. N. Am. Neuro-Ophthalmol. Soc. 2015, 35 (Suppl. S1), S38–S44.
- Wostyn, P.; Van Dam, D.; Audenaert, K.; Killer, H.E.; De Deyn, P.P.; De Groot, V. A new glaucoma hypothesis: A role of glymphatic system dysfunction. Fluids Barriers CNS 2015, 12, 16.
- Gallina, P.; Savastano, A.; Becattini, E.; Orlandini, S.; Scollato, A.; Rizzo, S.; Carreras, G.; Di Lorenzo, N.; Porfirio, B. Glaucoma in patients with shunt-treated normal pressure hydrocephalus. J. Neurosurg. JNS 2017, 129.
- Yablonski, M.E.; Ritch, R.; Pokorny, K.S. Effect of decreased intracranial-pressure on optic disk. In Investigative Ophthalmology & Visual Science; Lippincott-Raven Publ 227 East Washington Sq: Philadelphia, PA, USA, 1979; p. 165.
- Berdahl, J.P.; Fautsch, M.P.; Stinnett, S.S.; Allingham, R.R. Intracranial pressure in primary open angle glaucoma, normal tension glaucoma, and ocular hypertension: A case-control study. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5412–5418.
- Ren, R.; Jonas, J.B.; Tian, G.; Zhen, Y.; Ma, K.; Li, S.; Wang, H.; Li, B.; Zhang, X.; Wang, N. Cerebrospinal Fluid Pressure in Glaucoma. A Prospective Study. Ophthalmology 2010, 117, 259–266.
- Siaudvytyte, L.; Januleviciene, I.; Ragauskas, A.; Bartusis, L.; Meiliuniene, I.; Siesky, B.; Harris, A. The difference in translaminar pressure gradient and neuroretinal rim area in glaucoma and healthy subjects. J. Ophthalmol. 2014, 2014, 937360.
- Lindén, C.; Qvarlander, S.; Jóhannesson, G.; Johansson, E.; Östlund, F.; Malm, J.; Eklund, A. Normal-Tension Glaucoma Has Normal Intracranial Pressure: A Prospective Study of Intracranial Pressure and Intraocular Pressure in Different Body Positions. Ophthalmology 2018, 125, 361–368.
- Jóhannesson, G.; Eklund, A.; Lindén, C. Intracranial and Intraocular Pressure at the Lamina Cribrosa: Gradient Effects. Curr. Neurol. Neurosci. Rep. 2018, 18, 25.
- Wostyn, P.; De Groot, V.; Van Dam, D.; Audenaert, K.; Killer, H.E.; De Deyn, P.P. The Glymphatic Hypothesis of Glaucoma: A Unifying Concept Incorporating Vascular, Biomechanical, and Biochemical Aspects of the Disease. Biomed. Res. Int. 2017, 2017, 5123148.