Currently, polymers are competing with metals and ceramics to realize various material characteristics, including mechanical and electrical properties. Flame retardants can also be introduced to further reinforce the fire performance of polymers.
- flame retardants
- nanofillers
1. Introduction
According to the statistics from the National Emergency Management Agency of South Korea during the period of 2010 to 2020, the number of large-scale fires (standard: 5 deaths, 10 casualties, and $4 million of property damage) increased six-fold from 3 to 18, and the casualties (from 45 deaths in 2010 to 232 deaths in 2019) and property damage costs (from $5 million to $330 million) increased significantly as well. These fires were mainly caused due to electrical and mechanical faults, with unknown causes also accounting for a significant proportion of these large-scale fires [1][2][3][1,2,3]. Based on the fire statistics for 2019, burns, smoke, and inhalation of toxic gases were the main reasons for the casualties [4][5][6][7][4,5,6,7]. Thus, fire protection becomes crucial; however, it is significantly challenging. As shown in Figure 1, a typical fire scenario includes several processes. First, ignition, which is defined as the initiation of combustion, occurs; this is followed by fire growth, which is defined as the fire development stage during which the heat release rate and fire temperature increase. During the initial stage of a fire outbreak, the fire spreads quickly, and within a few minutes, the generated smoke and heat result in “flashover.” Once the fire has reached this stage, it is difficult to control the fire [8][9][10][11][12][8,9,10,11,12]. Since polymer materials are used for various applications, the incorporation of functional additives to polymer materials has attracted significant research attention [13][14][15][16][13,14,15,16]. In particular, the development of flame-retardant polymer materials has attracted attention toward managing the disadvantages of heat-sensitive polymers [17][18][19][20][21][22][23][17,18,19,20,21,22,23]. The poisonous gases released due to combustion, which is the secondary damage caused by fire, increase the harm done to humans; therefore, developing flame retardants and flame-resistant polymer materials is still crucial [24][25][26][24,25,26]. The typical characteristics of a fire include the following: i) Flame spread: The size of flame and/or the time it takes for the flame to cover a defined distance from the sample; ii) dripping: The presence of flame droplets that can ignite other objects; iii) heat release: Heat generated by the combustion of a sample in the room; and iv) the opacity and toxicity of the smoke, which are important for the evacuation of people trapped in the fire.
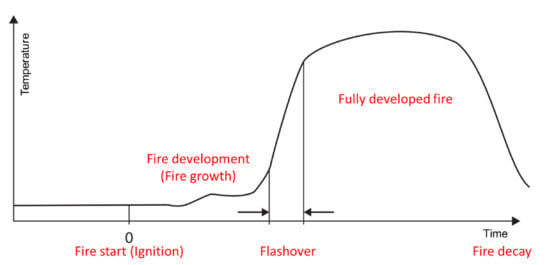
Figure 1.
2. Polymer Combustion
When exposed to sufficient heat, polymers gradually decompose and generate flammable gases that react with oxygen in ambient air to form an ignitable source. When the temperature is high enough for autoignition, ignition occurs either impulsively or at the flash point. Upon combustion, heat is released, some of which is transferred to the substrate, thereby promoting further decomposition. If there is enough heat to maintain the polymer decomposition rate such that the concentration of volatiles remains within the flammability limits, a self-sustaining combustion cycle will be established (Figure 2). Three elements, i.e., heat, oxygen, and fuel, are required to sustain the fire [8]. The heat source increases the temperature of the polymer, which depends on the strength of the heat source and inherent material properties. This temperature increase promotes the pyrolysis and formation of low-molecular-weight volatile species; a typical scheme for polymer decomposition with volatile species formation during pyrolysis is shown in Figure 3 [27]. When the volatile species combines with oxygen and the concentration reaches a critical level, the gaseous product (i.e., the mixture of fuels) ignites, and the resulting flame becomes a heat source for maintaining polymer decomposition, which is also known as the condensed phase. To suppress or reduce polymer fire, the fire cycle must be stopped by suppressing the heat, fuel, or combustion.
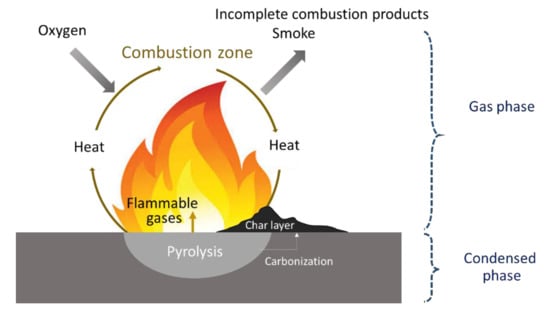
Figure 2.
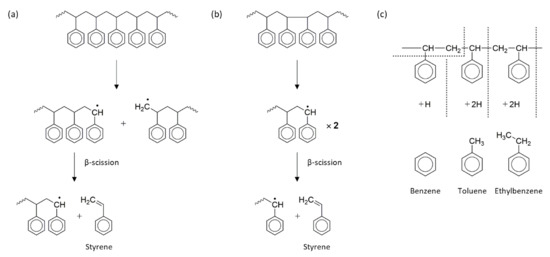
Figure 3.
a
b
c) plausible processes for the generation of volatile species such as benzene, toluene, and ethylbenzene [27][28].
Most natural polymer materials such as rubber and wood, are being replaced with synthetic polymers, and several organic polymer materials have been reported [15][29][30][31][32][33][34][15,29,30,31,32,33,34]. Synthetic polymer products, such as elastomers and plastics, can comprise one or more polymers and can contain other types of compounds, such as mineral fillers and dyes [15][35][36][37][38][15,35,36,37,38]. To reduce fire damage, polymer ignition delay is crucial, and it can be considered as an initial goal (Figure 4) [35]. As flame combustion is a gas-phase oxidation process, oxygen must be present in the atmosphere. Therefore, the polymer is decomposed before combustion occurs actively, since decomposition produces flammable volatile species that act as a fuel in the presence of oxygen. Table 1 shows that the combustion heats of organic synthetic polymers are greater than those of natural polymers, and the generated gas is toxic [35]. This increases the risk in the event of a fire, and thus the fire performance of organic polymers has attracted continuous attention [39][40][41][42][43][44][39,40,41,42,43,44]. Polymer flame-retarding methods include reactive and additives types, and the latter are further divided into organic and inorganic flame retardants [24][40][41][42][24,40,41,42].
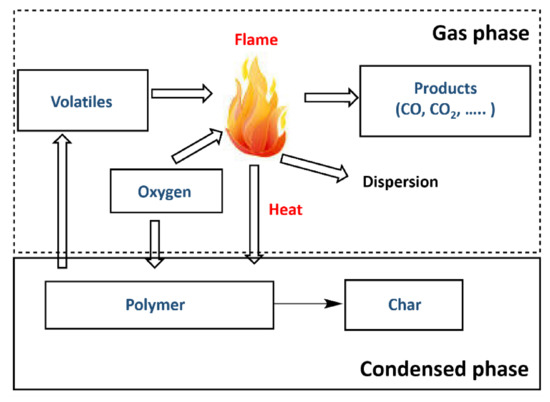
Figure 4. Combustion cycle of polymers. Reprinted with permission from Ref. [35]. Copyright 2016, MDPI AG.
Table 1.
| Polymer | Heat of Combustion (Δ | H | , kJ/g) |
|---|---|---|---|
| Polyethylene | 46.5 | ||
| Polypropylene | 46.5 | ||
| Polybutadiene | 45.2 | ||
| Polystyrene | 41.5 | ||
| Acrylonitrile butadiene styrene copolymer | 36.0 | ||
| Polycarbonate | 31.0 | ||
| Poly(methyl methacrylate) (PMMA) | 26.1 | ||
| Poly(vinyl chloride) | 24.7 | ||
| Polyethylene terephthalate | 22.2 | ||
| Cotton | 17.0 | ||
| Cellulose | 16.7 |
Polymer combustion begins with heat-induced decomposition (pyrolysis) of the solid polymer, which emits volatile organic gases that mix with oxygen and result in combustion [45][46][47][48][45,46,47,48]. The heat of combustion continues the pyrolysis process that maintains a positive feedback until the cycle is broken due to the lack of heat/fuel/oxygen (Figure 5) [49]. This occurs for all the polymer materials. Thermoplastic polymers tend to lead the additional process of flame spreading or propagation, instead of generating a pyrolysis gas directly from the sample surface to the condensed phase.
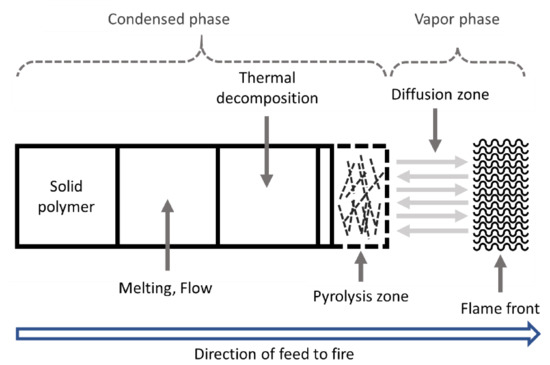
Figure 5.
During combustion, char-type flame retardants combine the fuel with non-pyrolytic carbon (char) to prevent fuel release and provide thermal insulation to the base polymer by forming a protective char layer [50][51][52][53][54][55][56][50,51,52,53,54,55,56]. In other words, the flame retardant causes charring on the polymer surface through dehydration of the flame retardant to generate double bonds in the polymer [57][58][59][60][61][62][57,58,59,60,61,62]. The carbon layer (charring) generated in this process contributes to the flame-retardant effect. Figure 6 shows an example of the charring process that can occur during the normal burning of polymers [63]. The char layer acts as a protective barrier, and during combustion, the char stability is enhanced by the decomposing polymer.
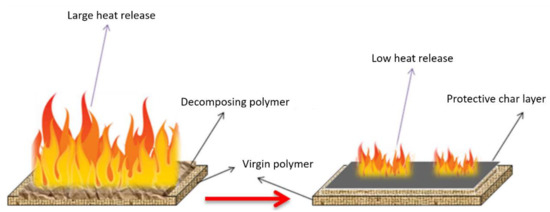
Figure 6. Schematic illustrating the stabilizing effect of char layer through modification of the heat release and consequent decomposition of virgin polymer during burning. Reprinted with permission from Ref. [63]. Copyright 2017, Springer-Verlag GmbH Germany.
