Alzheimer’s disease (AD) is the most common neurodegenerative disease worldwide. Histopathologically, AD presents with two hallmarks: neurofibrillary tangles (NFTs), and aggregates of amyloid β peptide (Aβ) both in the brain parenchyma as neuritic plaques, and around blood vessels as cerebral amyloid angiopathy (CAA). According to the vascular hypothesis of AD, vascular risk factors can result in dysregulation of the neurovascular unit (NVU) and hypoxia.
1. Introduction
The neurovascular unit (NVU) is defined as a complex functional and anatomical structure composed of: (a) neurons and interneurons, (b) glial cells such as microglia, astrocytes, and oligodendrocytes, (c) vascular cells such as endothelial cells, pericytes, and smooth muscle cells (SMCs), and (d) a basal lamina formed by brain endothelial cells and extracellular matrix (
) [1][2]. The NVU components are intimately linked to each other, enabling an efficient system for cerebral blood flow (CBF), maintenance of neuronal metabolic activity [3], and an effective blood-brain barrier (BBB). The BBB is a specialized structure in the cerebral vasculature formed by astrocytes, pericytes, and specialized junctions such as tight-junctions (TJs) and adherent-junctions (AJs) of endothelial cells [4]. The BBB serves to limit the entry of pathogens, toxic agents, and blood cells into the brain parenchyma [5]. The junctional components of the NVU are made by gap junctions and adhesion molecules such as integrins and cadherins [6]. This interplay facilitates the influx/efflux of Ca
) [1,2]. The NVU components are intimately linked to each other, enabling an efficient system for cerebral blood flow (CBF), maintenance of neuronal metabolic activity [3], and an effective blood-brain barrier (BBB). The BBB is a specialized structure in the cerebral vasculature formed by astrocytes, pericytes, and specialized junctions such as tight-junctions (TJs) and adherent-junctions (AJs) of endothelial cells [4]. The BBB serves to limit the entry of pathogens, toxic agents, and blood cells into the brain parenchyma [5]. The junctional components of the NVU are made by gap junctions and adhesion molecules such as integrins and cadherins [6]. This interplay facilitates the influx/efflux of Ca 2+
, K
+
, and the neuromodulatory action of ATP [7]. Each NVU component plays an active and specific role in maintaining the dynamic linkages reciprocally under physiological conditions [8]. Dysregulation of the NVU and BBB are critical pathophysiological events in neurodegenerative diseases, including Alzheimer’s disease (AD) [9].
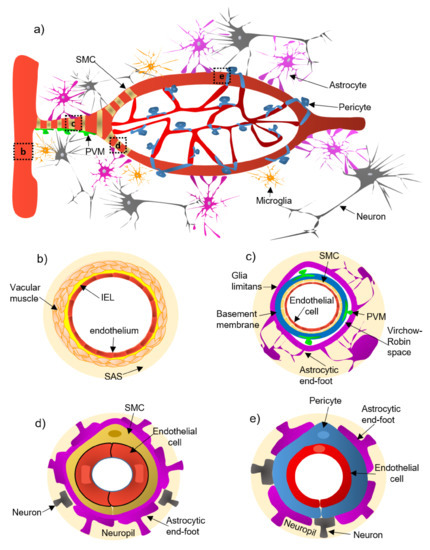
Figure 1.
Illustration of the constitutive cellular elements of the neurovascular unit (NVU) along the entire brain vasculature. Panel (
a
) shows a panoramic view of the vascular tree. Panels (
b
–
e
) depict the different levels of the vascular tree. Pial arteries (panel (
b
)) run along the brain surface and penetrate the parenchyma, narrowing and branching into penetrating arterioles (panel (
c
)), which form the intraparenchymal arterioles (panel (
d
)), and ultimately give rise to capillaries (panel (
e
)). Abbreviations: IEL, internal elastic lamina; PVM, perivascular macrophage; SAS, the subarachnoid space; SMC, smooth muscle cell.
AD is one of the most important neurodegenerative disorders worldwide and the leading cause of cognitive and functional decline in the elderly [10]. The classic neuropathological hallmarks of AD involve aggregates of tau and amyloid β-peptides (Aβ). Neurofibrillary tangles (NFTs) and hyperphosphorylated tau accumulate intracellularly and are typically accompanied by neuronal loss [11][12]. The second hallmark is the presence of amyloid β-peptide (Aβ) deposits in the brain parenchyma and around cerebral blood vessels as neuritic plaques (NPs) and cerebral amyloid angiopathy (CAA), respectively. CAA is characterized by Aβ deposition within the walls of cortical and leptomeningeal arteries and veins [13], which lead to NVU dysfunction [11][12]. CAA is universally found in AD brains [14] and has been linked to neuroinflammation, chronic hypoperfusion, ischemia, and loss of the blood vessel wall integrity and hemorrhage [15][16].
AD is one of the most important neurodegenerative disorders worldwide and the leading cause of cognitive and functional decline in the elderly [10]. The classic neuropathological hallmarks of AD involve aggregates of tau and amyloid β-peptides (Aβ). Neurofibrillary tangles (NFTs) and hyperphosphorylated tau accumulate intracellularly and are typically accompanied by neuronal loss [11,12]. The second hallmark is the presence of amyloid β-peptide (Aβ) deposits in the brain parenchyma and around cerebral blood vessels as neuritic plaques (NPs) and cerebral amyloid angiopathy (CAA), respectively. CAA is characterized by Aβ deposition within the walls of cortical and leptomeningeal arteries and veins [13], which lead to NVU dysfunction [11,12]. CAA is universally found in AD brains [14] and has been linked to neuroinflammation, chronic hypoperfusion, ischemia, and loss of the blood vessel wall integrity and hemorrhage [15,16].
2. Cellular and Structural Components of the NVU Along the Cerebrovascular Tree
An extensive system of arteries, arterioles, and capillaries, delivers oxygenated blood and nutrients to the brain. The pial arteries derived from arteries go along the brain surface and penetrate the parenchyma, branching into arterioles and capillaries [17]. The cellular NVU composition changes along the cerebrovascular tree ( a):
- (a)
-
(a) Pial arteries consist of multiple layers of SMCs, separated from the endothelium by a notable elastic lamina (
-
Pial arteries consist of multiple layers of SMCs, separated from the endothelium by a notable elastic lamina (b), and innervated by nerve fibers formed from sensory and peripheral autonomic ganglia [18]. The pial arteries penetrating the brain are surrounded by the subarachnoid space (SAS; b). Traditionally, the pial arteries have been associated with CBF and neuronal homeostasis [19].
b). Traditionally, the pial arteries have been associated with CBF and neuronal homeostasis [19].-
- (b)
-
(b) Penetrating arterioles have several thin SMC layers that become a single layer (
-
Penetrating arterioles have several thin SMC layers that become a single layer (c) [20]. The density of perivascular nerves is low at this level, and the elastic lamina becomes less prominent [20]. The perivascular space (also known as “Virchow-Robin space”) is delimited by the astrocytic end-foot (glia limitans) and the vascular basement membrane [21]. It comprises different cell types, including perivascular macrophages (PVMs), pial cells, Mato cells and mast cells, and collagen and nerve fibers [21] (c). The perivascular space is an exchange pathway for the glymphatic system. The glymphatic system has been defined as a network of perivascular pathways that favors the exchange of both solutes and liquids between the cerebrospinal fluid (CSF) and the interstitial compartments, promoting clearance of metabolites, proteins, and debris from the brain interstitium [22][23][24]. This clearance depends mainly on the aquaporin-4 water channels (AQP4), found in astrocytic end-feet [25]. It has been demonstrated that the pial and penetrating arterioles can regulate arterial tone by the extrinsic and intrinsic innervations, respectively [26].
c). The perivascular space is an exchange pathway for the glymphatic system. The glymphatic system has been defined as a network of perivascular pathways that favors the exchange of both solutes and liquids between the cerebrospinal fluid (CSF) and the interstitial compartments, promoting clearance of metabolites, proteins, and debris from the brain interstitium [22,23,24]. This clearance depends mainly on the aquaporin-4 water channels (AQP4), found in astrocytic end-feet [25]. It has been demonstrated that the pial and penetrating arterioles can regulate arterial tone by the extrinsic and intrinsic innervations, respectively [26].-
- (c)
-
(c) The intraparenchymal arterioles are formed when the arterioles invade deeper into the brain. Here, the glial limitans and the vascular basement membrane fuse, eliminating the Virchow-Robin space [21]. These arterioles have a single layer of SMCs, lack perivascular nerves, and are encapsulated by the astrocytic end-feet (
-
The intraparenchymal arterioles are formed when the arterioles invade deeper into the brain. Here, the glial limitans and the vascular basement membrane fuse, eliminating the Virchow-Robin space [21]. These arterioles have a single layer of SMCs, lack perivascular nerves, and are encapsulated by the astrocytic end-feet (d) [20]. Endothelial cells extend their protrusions to SMCs and connect through gap junctions [27]. They are related to functional hyperemia, which ensures a rapid increase in the CBF rate to activated brain structures [28].
d) [20]. Endothelial cells extend their protrusions to SMCs and connect through gap junctions [27]. They are related to functional hyperemia, which ensures a rapid increase in the CBF rate to activated brain structures [28].-
- (d)
-
(d) Capillaries are the smallest vessels in the brain and exchange molecules between blood and brain across the BBB [29]. Pericytes replace the SMCs, and mural cells are immersed in the endothelial basement membrane (
-
Capillaries are the smallest vessels in the brain and exchange molecules between blood and brain across the BBB [29]. Pericytes replace the SMCs, and mural cells are immersed in the endothelial basement membrane (e) [30]. The border of the capillaries is enveloped by astrocytic end-feet, and the neural processes can be adjacent to the capillary basal lamina (e) [31]. Pericytes and endothelial cells make direct interdigitated contacts where cytoplasmic protrusions (pegs) of one cell type insert into the opposing cell membrane (socket) of the other cell type [32].-
3. The Physiological Characteristics of the Blood-Brain Barrier (BBB)
The endothelial cells that make up the brain capillaries are tightly sealed and act as a barrier, referred to as the “blood-brain barrier” (BBB). The BBB is a specialized brain endothelial composition of the neurovascular system that is completely differentiated. Together with astrocytic end-feet, pericytes, and microglia, the BBB separates the circulating blood components from neurons [2][33] (
The endothelial cells that make up the brain capillaries are tightly sealed and act as a barrier, referred to as the “blood-brain barrier” (BBB). The BBB is a specialized brain endothelial composition of the neurovascular system that is completely differentiated. Together with astrocytic end-feet, pericytes, and microglia, the BBB separates the circulating blood components from neurons [2,33] ( e and
a).
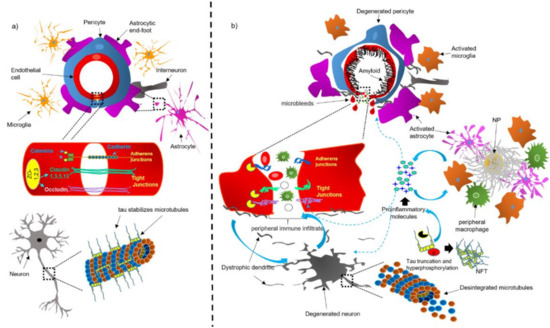
Figure 2.
Schematic representation of the NVU under physiological (
a
) and pathological (
b
) conditions. Abbreviations: NFT, neurofibrillary tangle; NP, neuritic plaque.
The nearness of the different cell types with one another in the NVU allows for an effective paracrine regulation, the maintenance of the BBB, and the normal functioning of the central nervous system (CNS), such as synaptic transmission and remodeling, neurogenesis, and angiogenesis in the adult brain [2]. The cell-to-cell hermetic contact confers to the BBB the properties of low paracellular and transcellular permeability and high transendothelial electrical resistance [29].
The firmly sealed endothelium limits the entry of most blood-derived particles into the brain unless they possess receptors or carriers (glucose, hormones, amino acids, and nucleotides) to be transported across the BBB [34]. The concentration gradient is the most considerable factor for carrier-mediated transport across the BBB and is preferentially influenced by the affinity, size, and physiochemical properties of each specific molecule [35]. The BBB excludes typically free exchange of solutes between blood-brain and brain-blood [36]. One exception is that of small lipid-soluble molecules <400 Da, which can cross the BBB via lipid-mediated diffusion [37], adsorptive endocytosis, and receptor-mediated endocytosis [38]. On the other hand, transendothelial passive diffusion enables the small influx of lipophilic and nonpolar molecules into brains across the lipid bilayer of endothelial cells [38].
Endothelial cells are typically connected at a junctional complex by the tight-junctions (TJs) and adherent-junctions (AJs) (
a) [39]. The TJs provide low paracellular permeability, high electrical resistance [40], and binding to the cytoskeleton. Claudin ( a), a transmembrane protein of 207–305 amino acids, is the main structural component of the TJs [41]. Different claudin isoforms are expressed in brain endothelial cells, such as claudin-1, -3, -5, and -12; claudin-5 being the most highly expressed in these cells [12]. Occludin ( a), another tetraspan transmembrane protein of 522 amino acids of TJs, regulates BBB integrity and permeability [42]. The transmembrane TJ proteins are linked to the actin cytoskeleton through the zonula occludens-1 (ZO-1; a), a membrane-associated protein [43]. Together, these complex of TJ proteins decrease the paracellular diffusion and limit transcellular activity [12].
The AJs are typically found intermingled with the TJs and contribute to regulating the BBB permeability and leukocyte extravasation [44]. Vascular endothelial (VE)-cadherin is an endothelial-specific integral membrane protein, connected to the cytoskeleton via catenins ( a). The localization and expression of β-catenin, α-catenin, and p120cas, are essential for the functionality of AJs [40]. Neutrophils and lymphocytes can ingress from the blood into the brain regulated by BBB and low levels of leukocyte adhesion molecules (LAMs) [45]. Therefore, this property may prevent access of immune cells from blood to the brain, affording immunologic benefit in the CNS [45].
BBB integrity is essential for controlling the molecular composition of brain interstitial fluid (ISF), which is indispensable for correct information processing, synaptic operating, functioning, and neuronal connectivity [34]. BBB breakdown could trigger an increase in vascular permeability, reduce CBF and impaired hemodynamic responses [17]. Therefore, BBB dysfunction could facilitate the entry of toxic blood-derived molecules and cells and trigger an immune response associated with neuroinflammation, which ends in degeneration of the NVU ( b and
steps 2c, 3 and 4).
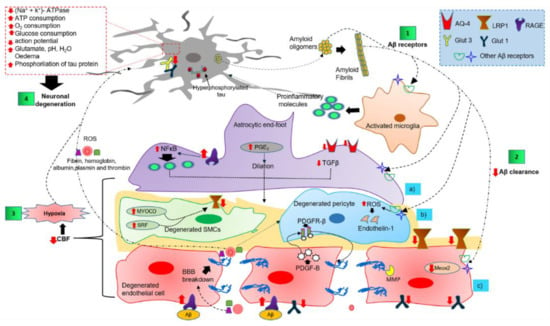
Figure 3.
Overview of the complex cell–cell signaling in the NVU, triggered by extracellular and vascular Aβ deposits. Step 1 shows the interaction of Aβ oligomers and fibrils with NVU cells through several receptors. Step 2 illustrates the altered Aβ clearance that triggers NVU dysfunction. Step 3 shows the NVU cell dysfunction as the causative agent of cerebral hypoxia. Step 4 shows cerebral hypoxia, neuroinflammatory environment, and peripheral blood infiltrate as the promoting causes of neuronal degeneration. Abbreviations: AQ-4, aquaporin-4; Aβ, amyloid peptide; BBB, blood-brain barrier; CBF, cerebral blood flow; GLUT, glucose transporter; LRP1, low-density lipoprotein receptor-related protein 1; MEOX2, mesenchyme homeobox 2; MMP, matrix metalloproteinase; MYOCD, myocardin; NFκB, nuclear factor kappa light chain enhancer of activated B cells; PDGF-B, platelet-derived growth factor subunit B; PDGFRβ, platelet-derived growth factor receptor β; PGE
2
, prostaglandin E2; TGFβ, transforming growth factor-beta; RAGE, receptor for advanced glycation end products; ROS, reactive oxygen species.