The world’s human population continues to increase, posing a significant challenge in ensuring food security as soil nutrients and fertility decrease with time. Thus, there is a need to increase agricultural productivity to meet the growing population's food demands. A high level of chemical fertilizers to increase food production is damaging ecological balance and human health. It is becoming too expensive for many farmers to afford. The exploitation of beneficial soil microorganisms as a substitute for chemical fertilizers in food production is one potential solution to this conundrum. Microorganisms, such as plant growth-promoting rhizobacteria and mycorrhizal fungi, have demonstrated their ability to formulate biofertilizers in the agricultural sector, providing plants with nutrients required to enhance their growth, increase yield, manage abiotic and biotic stress, and prevent phytopathogens attack. Beneficial soil microbes have been reported to produce some volatile organic compounds beneficial to plants. The amendment of these microbes with locally available organic materials and nanoparticles is currently used to formulate biofertilizers to increase plant productivity.
- beneficial microorganisms
- biofertilizers
- crop production
- soil fertility
- sustainable agriculture
1. Introduction
According to the Food and Agriculture Organization (FAO) of the United Nations, the world's population is expected to increase to more than nine billion by 2050, a third more people to feed than today. It is, therefore, necessary to dramatically increase agricultural production by managing the rhizosphere in a relatively short period [1] to ensure food security. Some factors are necessary to meet this goal, including the right environmental conditions and availability of fertile soil [2] conditions that are becoming rarer with time. From the middle of the 20th century until date, chemical fertilizers have helped feed the world’s population. This has been done through the provision of the required nutrients, such as phosphorus (P), nitrogen (N), and potassium (K), to plants. About 53 billion tons of NPK fertilizers are used yearly to supplement the number of nutrients needed for plant growth and yield performance [3]. Unfortunately, only a small percentage of these nutrients are used by plants, while a greater percentage is precipitated by metal cations present in the soil. Moreover, the extensive and inappropriate use of chemical fertilizers results in environmental issues that are a major concern to farmers, furthering the argument for introducing agricultural practices that do not harm the environment [4]. Scientists have begun to direct their interests towards ensuring agrarian sustainability using beneficial soil microorganisms instead of chemical fertilizers and pesticides [5].
Rhizosphere management can be defined as improving the nutrient efficiency in the soil to enhance the nutrient needed for plant growth and improve plant yield [6]. Beneficial soil microorganisms enhance the management of the rhizosphere through different mechanisms that are multidimensional. These include the following: production of siderophore, nitrogen fixation, lytic acid production, production of hydrogen cyanide, phosphate solubilization, and production of indole acetic acid [7][8]. The mechanisms of action of these beneficial microorganisms play a crucial role in improving soil fertility, plant growth, and yield.
Many beneficial soil microorganisms have been isolated for their potentials in the management of the rhizosphere to enhance plant yield [9] and are currently used in biotechnology as tools to improve food security and agricultural sustainability. Currently, mycorrhiza fungi and plant growth-promoting rhizobacteria (PGPR) are perceived by soil researchers as microorganisms that play vital roles in ensuring nutrients availability in the soil to enhance plant growth and increase yield. Biofertilizers' application is gaining more awareness since it is an environmentally friendly and cost-effective means of enhancing crop productivity and soil fertility [10]. Microbial biofertilizers consist of viable cells of beneficial microorganisms, with plant growth-promoting potentials that interact with the rhizosphere or endosphere of plants by improving soil fertility and stimulating nutrient uptake to increase yield [11]. Biofertilizers' application reduces the high cost of purchasing chemical fertilizers and addresses the world’s demand for green technology for crop production [12]. Thus, this review focuses on the rhizosphere management to improve plant growth and yield through the application of PGPR and mycorrhizal fungi in the formulation of cost-effective and ecologically friendly microbial biofertilizers.
2. Categories of Microorganisms Used in the Production of Biofertilizers
2.1. Nitrogen-Fixing Microbes
Microorganisms that belong to the family Rhizobiaceae are made up of different genera, such as Rhizobium, Azorhizobium, Bradyrhizobium, Mesorhizobium, and Sinorhizobium, are known to be the best symbiotic nitrogen fixers and live in the plant root noodles (Figure 1). Rhizobium, in the root noodle, fixes atmospheric nitrogen in leguminous plants. Nitrogen is used by the plant to synthesize vitamins, amino acids, nucleic acids, and other nitrogenous compounds. All nitrogen-fixing microorganisms use the same enzyme–nitrogenase [13]. The role played by Rhizobia in nitrogen fixation makes leguminous plants less dependent on the application of chemical nitrogen fertilizers and is the key success for the crop rotation strategy for sustainable agriculture.
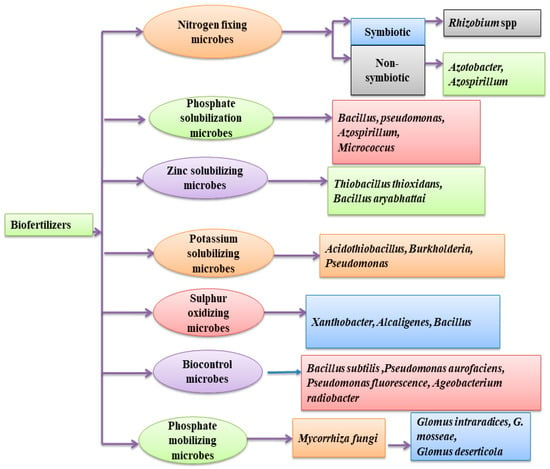
Figure 1. Schematic diagram of categories of microorganisms used as biofertilizers.
Nodule formation is enhanced by the low availability of nitrogen, but microorganisms that produce an enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase, have the potential to degrade 1-aminocyclopropane-1-carboxylate before its conversion to ethylene [14][15] and may also enhance the formation of a nodule. Such formation is part of a common strategy developed by leguminous plants and Rhizobiaceae bacteria to decrease the concentration of oxygen to which the nitrogenase is exposed due to the inhibitory effect of oxygen on nitrogenase activity. However, there are other nitrogen-fixing microorganisms, such as those of the Acetobacter genus, able to fix nitrogen even under aerobic conditions.
Certain strains of Azotobacter (Azotobacteriaceae family) have the potential to colonize the roots of sugarcane, coffee, cotton, wheat, rice, and vegetables [14][16]. Co-inoculation of wheat plants with specific strains of Azotobacter and Pseudomonas increases grain yield, protein content, and harvest index compared to uninoculated plants, which allowed a decrease in the application of chemical fertilizer in the field by 25–50% [17]. Azotobacter is an example of a nitrogen-fixing bacteria genus, able to fix nitrogen under aerobic conditions and can act as a biocontrol agent. Azotobacter indicum have been reported by Mahanty et al. [12] to have fungicide properties.
Several species of Azospirillum belonging to the family Rhodospirillaceae (A. zeae, A. thiophilum, A. rugosum, A. picis, A. oryzae, A canadense, A. mazonense, and A. melinis) have been found associated with grass rhizosphere [8] while fixing nitrogen. Plant inoculation with Azospirillum strains promotes plant growth and yield by causing changes in the cell wall elasticity or the morphology of the root, or both through the production of phytohormones (auxin) [18].
2.2. Phosphorus Solubilizing Microbes
Phosphorus is a macronutrient, and its low availability severely limits plant development and productivity. In most situations, the presence of phosphorus available in the soil is at high concentrations as phosphate, which may be in an organic or inorganic form. Only a small fraction of inorganic phosphate is available to the biosphere in the soil solution; most inorganic phosphate is immobilized in insoluble salts. Phosphorus solubilization involves local acidification or alkalinization and has been observed in some Pseudomonas species, Cyanobacteria, and Bacillus isolated from the rhizosphere of plants (Table 1).
Organic phosphate is the largest pool of soil phosphate. Still, organic phosphate compounds tend to be complex (nucleic acids, phospholipids, etc.) and have to be transformed by microorganisms before they can be absorbed by plants [19]. Hence, the phosphorus mineralization process in the soil involves the production of enzymes, such as phosphatases and phytases [20]. Phosphate solubilizing and mineralizing characteristics are found in some Pseudomonas, Cyanobacteria, and Bacillus (Table 1).
Table 1. Rhizobacteria used in the production of biofertilizer, biocontrol traits, and their effect on plant productivity
Potassium is an essential macronutrient regulating many enzyme activities, including amylases (enzymes involved in starch degradation), which are involved in the coordination of root shoot ratio [15]. An insufficient supply of potassium leads to poor development of the plant root, an increase in the plant's susceptibility to pathogens, and a reduction in plant growth and yield.
A large number of potassium solubilizing microorganisms live in the soil and have been reported in different studies [51]. These include some bacteria, such as Bacillus mucilaginous, Azotobacter chroococcum, and Rhizobium spp., which have been reported for potassium solubilization, resulting in increased maize, chili, cotton, pepper, sorghum, and wheat productivity [52]. Recently, the inoculation of wheat grown with a potassium solubilizing strain of Bacillus edaphicus was reported to have shown a great increase in roots and shoots growth when compared to uninoculated plants [33].
The production of organic acids is among the mechanisms used by potassium solubilizing microorganisms. This makes its usage in agriculture the best strategy for promoting plant productivity and enhancing soil fertility. The inoculation of plants with potassium solubilizing microorganisms increases the uptake of potassium, plant yield, and growth.
2.4. Phosphorus Mobilizing Microbes
Endomycorrhizal fungi are critical actors for improving phosphorus bioavailability (Figure 2), and some genera (Scutellospora, Glomus, Acaulospora, and Gigaspora) are already in use as biofertilizers. Since the fungal hyphae can penetrate the soil pores, sites where the root system cannot reach, the mycorrhizal plant root can efficiently explore a bigger soil volume than non-mycorrhizal plants [53]. Recently, the use of Glomus mosseae as a biofertilizer was reported [54] to increase the shoot length, root dry weight, and root length in wheat, and, at the same time, mycorrhizal hyphae also contribute to an increase in the soil structure [55]. The application of mycorrhizal fungi in agriculture (Table 2) is low cost and eco-friendly. It increases plant yield when compared to the cost of purchase of chemical fertilizers without any negative effect on the environment [56]. However, the beneficial role played by arbuscular mycorrhizal fungi to plants is negatively affected by tillage and the application of chemical fertilizers or pesticides (fungicides in particular). The application of arbuscular mycorrhizal fungi towards ensuring sustainable agriculture is gaining more awareness each day with their role in improving plant health, productivity, and soil fertility.
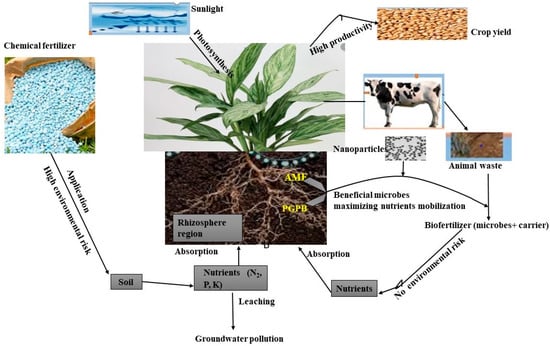
Figure 2. Overview of rhizosphere as the bottleneck in controlling nutrients uptake by plants through the application of chemical and biofertilizer; Arbuscular mycorrhiza fungi (AMF), Plant growth-promoting rhizobacteria (PGPR).
Table 2. Contribution of arbuscular mycorrhizal fungi to plant growth promotion and soil nutrients
| Mycorrhizal Fungi | Plants | Effect on Plant | Effect on Soil | References |
|---|---|---|---|---|
| Glomus versiforme Glomus mosseae |
Tomato | Promotes growth and yield under water stress and more efficient conditions | Increases phosphorus concentration in the soil | [57] |
| Glomus etunicatum | Maize | Improves chlorophyll content and nutrient uptake in maize | Increases soil quality | [58] |
| Acaulospora lacunosa | Strawberry | Enhances nutrient uptake in strawberry | Increases soil nutrient for horticultural crops productivity | [59] |
| Rhizophagus irregularis | Wheat | Improves tolerance to stress, enhances plant growth, and increases seed yield | Increases soil nutrient needed for wheat production | [60] |
| R. irregularis | Maize | Enhances tolerance to salt stress, improves growth parameters | Reduces the concentration of salt in the soil for better plant development | [61] |
| G. mosseae and G. geosporus | Strawberry | Enhances growth and improves its tolerance to water stress | Increases soil nutrient to enhance its colonization on the plant root system | [62] |
| Rhizophagus irregularis | Tomato | Protects plants against pathogens (Sclerotinia sclerotiorum) and improves nutrient uptake in plants | Increases soil micronutrient, triggers the defense of the plant against pathogens | [63] |
| Glomus deserticola | Snapdragon | Increases the total dry matter, chlorophyll content and improves Snapdragon tolerance to water stress | Increases soil nutrients needed for plant growth promotion | [64] |
| Glomus spp. and Mortierella spp. | Seashore mallow | Increases shoot and root weight under salt stress | Increases soil nutrient and enhances its absorption by plants | [65] |
| Glomus versiforme | Mentha arvensis L. | Increases dry weight and improves nutrient uptake in salt stress conditions | Increases soil nutrient and enhances its absorption by the plant to enhance its tolerance to salinity | [66] |
| Microbial Strains | Plant Growth-Promoting Traits | Biocontrol Traits | Effect on Plant Productivity | References |
|---|---|---|---|---|
| Bradyrhizobium sp. | Production of siderophore, production of indole acetic acid, nitrogen fixation, and phosphate solubilization | Production of antibiotics, secretion of an enzyme that can degrade the cell wall of plant–pathogen, production of hydrogen cyanide and, production of siderophore | Increases growth parameters and seed yield in mungbeans plant | [21][22] |
| Rhizobium meliloti | Production of siderophore and nitrogen fixation | Production of antibiotics against phytopathogens and production of chitinases | Increases peanuts growth, yield attributes, quality of pods, and efficiency in the use of nitrogen | [23][24] |
| R. leguminosarum | Phosphate solubilization | Production of antibiotics, secretion of an enzyme that can degrade the cell wall of plant pathogens and enhances the production of phytoalexins in plant | Increases growth of soybean and yield performance under drought stress | [25] |
| Bacillus spp. | Production of phytohormone, such as auxin, phosphate solubilization | Formation of endospore and biochemical compound against phytopathogens, induces systemic resistance and competition in plant | Increases strawberry fresh and dry weight parameters, increases yield over the control plant | [26][27] |
| Chryseobacterium sp. | Production of siderophore, phosphate solubilization | Production of proteases | Increases grain yield, shoot mass, and nodule mass in chickpea | [10][28][29] |
| Herbaspirillum spp. | Synthesis of indole acetic acid, nitrogen fixation | Production of siderophore | Enhances mineral uptake in maize plant and increases yield | [30][31][32] |
| Paenibacillus glucanolyticus | Synthesis of indole acetic acid | Production of chitinases and glucanases | Increases tissue dry weight and nutrient uptake in black pepper | [33][34] |
| Streptomyces spp. | Production of siderophore and synthesis of indole acetic acid | Production of glucanases | Increases tomato growth parameter and modulates metabolic activity | [35] |
| Burkholderia spp. | Solubilization of phosphate | Production of antibiotic pyrrolnitrin | Increases fenugreek growth and yield performance | [36][37] |
| Athrobacter | Solubilization of phosphate | Production of chitinases | Increases broccoli growth and yield | [38][39] |
| Phyllobacterium | Production of siderophore | NA | Increases grain yield in sorghum | [40][41] |
| Acinetobacter spp. | Production of ACC deaminase, Indole acetic acid synthesis, and phosphate solubilization | Production of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase | Promotes wheat growth in a greenhouse experiment | [42][43][44] |
| Acidothiobacillus ferooxidans | Potassium solubilization | NA | Increases pumpkin growth parameters, yield, and oil composition | [33][45] |
| Enterobacter cloacae | Nitrogen fixation, phosphate solubilization, siderophore production | Production of the lytic enzyme for chitinolytic activity, production of ACC deaminase | Enhances potato growth and promotes yield performance | [46][47] |
| Erwinia | Phosphate solubilization | Ethylene synthesis | Promotes growth and yield parameters in wheat | [48][49] |
| Pseudomonas spp. | Production of ACC deaminase phosphate solubilization, ammonia production, production of IAA | Production of hydrogen cyanide, siderophore production, production of cell wall degrading enzymes, such as chitinase and laminarinase, production of ACC deaminase, quorum sensing, and quenching | Enhances growth and yields in tomato plants | [10][50] |
2.3. Potassium Solubilizing Microbes
2.5. Sulphur Oxidizing Microbes
Sulphur is an essential macronutrient needed by plants in high concentration. It is part of some amino acids, such as cysteine, cystine, and methionine. It is among the components that regulate enzyme activity in plants, such as superoxide dismutase, ascorbate peroxidase, monodehydroascorbate reductase, dehydroascorbate reductase, and glutathione reductase. A deficiency in sulphur in plants results in low nitrogen metabolism, which causes chlorosis, low lipid percentage, and low plant growth and yield [67]. In the soil, sulphur exists in two forms: organic and inorganic, although the inorganic form of sulphur is primarily absorbed by plants (i.e., SO42−). Conversion of organic into inorganic sulphur forms may be carried out by sulphur-oxidizing microbes belonging to the genera Xanthobacter, Alcaligenes, Bacillus, and Pseudomonas. Some plant growth-promoting activities of sulphur-oxidizing microorganisms have been reported [33]. Pourbabaee et al. [68] reported the positive effect of Thiobacillus spp. on maize plants by increasing plant height, yield, and nitrogen uptake. Similarly, the positive effect of sulphur-oxidizing microorganisms on garlic plants was reported by Hejazirad et al. [69] to increase plant height, fresh and dry leaf mass, and bulb weight and diameter. Recently, the application of sulphur-oxidizing microorganisms has been recommended in the formulation of biofertilizer for onion, oats, ginger, grape, garlic, and cauliflower under alkaline soil conditions [70][71].
2.6. Zinc Solubilizing Microbes
Zinc is an essential micronutrient. The results of zinc deficiency in plants are reduction in leaf size, chlorosis, increase in plant susceptibility to heat, light stress, and pathogenic attack [72]. The application of Zn fertilizers has been suggested to pose a threat to the environment [73]. Thus, the application of zinc solubilizing microorganisms as an alternative to Zn supply is gaining traction. Several strains of Zn solubilizing microorganisms have been applied in the production of biofertilizers. These include Pseudomonas spp., Rhizobium spp., Bacillus aryabhattai, Thiobacillus thioxidans, and Azospirillum spp. [74]. Solubilization of Zn by microorganisms depends on both soil pH and capacity of cation exchange. Application of Bacillus spp. AZ6, as a Zn solubilizing biofertilizer on maize, was reported by Hussain et al. [75] to have a positive impact on total maize biomass and increase plant physiology, chlorophyll content by 90%, and yield when compared to uninoculated plants. Similarly, the application of Bacillus aryabhattai increased Zn uptake in maize, resulting in better growth and mitigation of yield loss in maize when compared to uninoculated plants [76]. Moreover, the effect of zinc solubilizing bacteria Rhizobium, Azospirillum, and Pseudomonas was reported to increase the zinc content in wheat when evaluated at different growing stages [77]. The effect of inoculation of these microorganisms enhances uptake of nutrients and production of the wheat plants with better quality. Additionally, the effect of zinc solubilizing bacterium Bacillus megaterium was recently reported by Bhatt and Maheshwari [78] to increase growth parameters resulting in maximum zinc content in Capsicum annuum L. fruit.
3. Beneficial Role of Biofertilizers on Plant Yield, Photosynthesis and Soil Nutrient
Biofertilizers are products composed of viable strains of microorganisms used to enhance plant growth without causing harm to human health or the environment [79]. Examples of microorganisms used in the production of biofertilizers that can increase plant growth and yield are nitrogen-fixing microbes, phosphorus solubilizing microbes, sulphur solubilizing microbes, mycorrhizal fungi, and potassium solubilizing microorganisms [80].
Recent research has revealed the effect of Rhizobium leguminosarum, Rhizobium spp. IRBG 74, and Bradyrhizobium spp. IRBG 271 increases plant biomass, yield, and chlorophyll content in plants compared with uninoculated plants. The highest increase was recorded with the IRBG strains, which showed a 14% increase when compared to uninoculated plants [81]. Similarly, certain Rhizobia strains can increase the surface area, photosynthetic rate, water uptake capacity, yield, and stomatal conductance of inoculated plants [82].
Furthermore, inoculation of a consortium of bacteria, namely, Pseudomonas, Bacillus lentus, and Azospirillum brasilense, was reported to increase chlorophyll content in plants and the expression of antioxidant enzymes under stress conditions [83]. Khalid et al. [84] found that biofertilizers' application on spinach increases growth, chlorophyll content, antioxidant activity, yield, and phenolic compounds. The total phenolic compounds were reported to be 58% higher when compared to uninoculated spinach. Similarly, Arora et al. [85] reported an increase in growth, yield, phenolic compounds, anthocyanins, and carotenoid content of lettuce when inoculated with Azotobacter chroococcum and Piriformospora indica. Kapoor and Singh [86] examined the biosynthesis of antioxidants by arbuscular mycorrhizal fungi. Likewise, Hassen et al. [87] reported an 80% increase in soybean yield when inoculated with nitrogen-fixing Rhizobium and Bradyrhizobium. Production of secondary metabolites, such as tannins, ortho-dihydroxy, and flavonoids, had also been reported in Begonia malabarica and Calamus thwaitesii, after being inoculated with Glomus mosseae , Trichoderma viride, and Bacillus coagulans [88].
The effect of biofertilizer in increasing plant growth and increasing plant yield for more food production has also been attached to its application. This is evident in research conducted by Dicko et al. [89], who reported that biofertilizer made from plant growth-promoting Actinomycetes (Actinomycetes sp. H7, O19, and AHB12) improved maize yield. Data obtained for the study revealed that the highest yield performance was recorded in biofertilizer made from a combination of O19 and AHB12, with a yield increase to 311.5 g for 1000 seeds compared to 178.28 g for the control plant. Recently, the effect of biofertilizer made from a plant growth-promoting Bacillus pumilus strain TUAT-1 was evaluated on two forage rice genotypes. The result obtained revealed that biofertilizer made from the Bacillus species increased rice productivity when compared to uninoculated [90]. Additionally, the application of biofertilizer in increasing maize growth and yield performance was reported by Fathi [91]. In the study, biofertilizer formulated using phosphate solubilizing bacteria was reported to enhance maize growth and yield when compared to uninoculated control.
More importantly, soil nutrients are reduced as a result of different activities that occur in the soil, which include runoff, bush burning, and leaching of agricultural soil. Nutrients in the soil migrate to the water body through runoff caused by rainfall, where it causes eutrophication and contamination of the water body [92]. This causes a major threat to the natural environment. Thus, the application of nutrient-rich biofertilizer made from plant growth-promoting microorganisms that have the potentials, such as nitrogen fixation, potassium solubilization, and phosphate solubilization, are essential in the recovery of soil nutrients to enhance plant growth and yield performance [93].
4. Formulation of Biofertilizers for the Management of the Rhizosphere
Formulation is a crucial step in the production of a biofertilizer since it has to maintain the viability of the microorganism used while maintaining its activity at low levels [94]. The formulation process involves preparation of inoculum, inclusion of additives, selection of the best carrier, sterilization of carrier material, scaling up, good quality control measures, and adequate packaging with the best delivery method (Figure 3). Formulation of microbial biofertilizer is regarded as a mixture that comprises one or more viable (active) strains of microorganisms aimed at improving plant metabolic activities at the site of application and is regarded as an alternative approach to chemical fertilizers [74]. Formulation aims to provide long shelf life to microorganisms. The carrier material serves as a support for the proliferation of microorganisms and ensures that the microorganism establishes itself with the plant. Additives protect the formulation from any unfavorable environmental conditions and improve the properties of the formulation [95]. Scaling up provides optimum growth conditions for the proliferation of the microbe used in the formulation process [96].

Figure 3. Simplified flow chart for the production of biofertilizer.
A good biofertilizer formulation must not be toxic or pollutant, should be economically viable (preferentially made from inexpensive materials), must permit nutrient uptake by the plant, increase plant yield, have a long shelf life, and remain viable under unfavorable conditions [15][97].
Additionally, the important issue to tackle in the production of biofertilizers for widespread use is the production of large quantities of pure inocula that have a high level of infectivity. Major aspects of the inoculation technology of plant growth-promoting microorganisms are the use of a good strain of microorganisms with plant growth-promoting functional genes for the preparation of inoculum, selection of an appropriate carrier, and the use of an appropriate method of delivery.
Furthermore, the selection of a viable strain of microorganism is important in this step (Table 2); once this is done, the production process, using standardized industrial methods, can follow. The cost of production of commercial biofertilizers is a significant constraint, and there are different carriers used in the production of biofertilizers (Table 3). Thus, the need to use some organic matter that is cheap and readily available arises; materials that have been used successfully include whey, water sludge, animal waste, and compost [98]. An alternative approach to reduce the cost of producing biofertilizer is to use residues from agro-industries that are enriched with rock phosphate. During the process of composting, microorganisms that can produce organic acid, improve phosphate solubilization activity are added to the carrier to ensure that the nutrients are made available to the plant [99].
Table 3. Classification of carrier materials for the production of biofertilizer.
| Categories of Carrier Material | Carrier Materials | References |
|---|---|---|
| Natural materials | Peat, lignite, coal, clay, and organic soil | [100] |
| Inert materials | Talc, vermiculite, perlite kaolin, bentonite, silicate, rock phosphate, calcium sulfate, and zeolite | [101][102] |
| Synthetic polymers | Polyacrylamide, polystyrene, and polyurethane | [103] |
| Natural polymers | Xanthan gum, carrageenan, agar agar, and agarose | [104] |
| Organic materials | Charcoal, biochar, composts, farmyard manure, sawdust, maize straw, vermicompost, cow dung, corn cob, and wheat husk | [105][106][107][108] |
| Agro-industry by-product | Sludge ash, jagerry | [100][109] |
Currently, inoculation of crops with mycorrhizal fungi as biofertilizers is becoming more common because of the reduction in the population of indigenous mycorrhiza fungi in the soil through the application of chemical fertilizer [110]. However, in selecting appropriate mycorrhizal fungi, it is essential to select high-quality mycorrhiza fungi, which will be able to colonize plant roots, act in the presence of bacteria, and have a long shelf life in the field and greenhouse [111]. The easiest way of propagating arbuscular mycorrhizal fungi is through the propagation of a viable spore using sterile soil and a suitable host plant. This requires the cultivation of inoculated host plants in sterile soil and arbuscular mycorrhizal fungi spores being allowed to develop and propagate within the host plant [112]. This method of producing inoculum is referred to as soil-based inoculum, which is the most common method used in the multiplication of arbuscular mycorrhiza spore.
The successful use of arbuscular mycorrhizal fungi depends on the strain utilized, the host plant, and the substrate used for propagation. More importantly, host plants that are commonly used in the propagation of arbuscular mycorrhizal fungi are sorghum and maize because of their high infectivity by mycorrhizal fungi. Roots and soil containing mycorrhizal fungi are harvested at the growing cycle, dried, and used as an inoculum.
Recently, new technology has been introduced in the formulation of biofertilizer, which involves the amendment of plant growth-promoting microbes with nanoparticles [113]. This technique involves the use of nanoparticles made from organic or inorganic material with at least 100 nm in size. In agriculture, this technique is referred to as the agro–nanotechnology approach. Plant growth-promoting microbes are integrated into the nanostructure to enhance yield performance in plants [114]. The formulation of nano-biofertilizer has efficiently enhanced agricultural productivity by increasing high retention in soil moisture content and increasing essential nutrient due to the direct and indirect effects of nanomaterial coating on plant growth-promoting microorganisms. Its application has been reported to increase yield performance in cereal and leguminous plants by stimulating the germination potency in plants [115].
References
- FAO; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2018: Building Climatic Resilience for Food Security and Nutrition; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018.
- Glaser, B.; Lehr, V.-I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Scient. rep. 2019, 9, 9338.
- FAO; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2017: Building Resilience for Peace and Food Security; FAO: Rome, Italy, 2017.
- Babalola, O.O. Beneficial bacteria of agricultural importance. Biotechnol. Lett. 2010, 32, 1559–1570.
- Yadav, K.K.; Sarkar, S. Biofertilizers, Impact on Soil Fertility and Crop Productivity under Sustainable Agriculture. Environ. Ecol. 2019, 37, 89–93.
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2020, 242, 126626.
- Cruz, C.; Lips, S.H.; Martins-Loução, M.A. Interactions between nitrate and ammonium during uptake by carob seedlings and the effect of the form of earlier nitrogen nutrition. Physiol. Plant. 1993, 89, 544–551.
- Babalola, O.O.; Glick, B.R. The Use of Microbial Inoculants in African Agriculture: Current Practice and Future Prospects. J. Food Agric. Environ. 2012, 10, 540–549.
- Reed, M.; Glick, B.R. Applications of plant growth-promoting bacteria for plant and soil systems. In Applications of Microbial Engineering; Taylor and Francis: Enfield, CT, USA, 2013; pp. 181–229.
- Glick, B.R. Introduction to plant growth-promoting bacteria. In Beneficial Plant-Bacterial Interactions; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–37.
- Okur, N. A review- biofertilizers- power of beneficial microorganisms in soils. Biomed. J. Sci. Tech. Res. 2018, 4, 4028–4029.
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Poll. Res. 2017, 24, 3315–3335.
- Saha, B.; Saha, S.; Das, A.; Bhattacharyya, P.K.; Basak, N.; Sinha, A.K.; Poddar, P. Biological nitrogen fixation for sustainable agriculture. In Agriculturally Important Microbes for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2017; pp. 81–128.
- Ma, W.; Penrose, D.M.; Glick, B.R. Strategies used by rhizobia to lower plant ethylene levels and increase nodulation. Can. J. Microbiol. 2002, 48, 947–954.
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agric. Biotechnol. 2019, 23, 101487.
- Wani, S.A.; Chand, S.; Wani, M.A.; Ramzan, M.; Hakeem, K.R. Azotobacter chroococcum—A potential biofertilizer in agriculture: An overview. In Soil Science: Agricultural and Environmental Prospectives; Springer: Berlin/Heidelberg, Germany, 2016; pp. 333–348.
- Zaidi, A.; Khan, M.S.; Saif, S.; Rizvi, A.; Ahmed, B.; Shahid, M. Role of nitrogen-fixing plant growth-promoting rhizobacteria in sustainable production of vegetables: Current perspective. In Microbial Strategies for Vegetable Production; Springer: Berlin/Heidelberg, Germany, 2017; pp. 49–79.
- Pereyra, M.A.; Creus, C.M. Modifying the rhizosphere of agricultural crops to improve yield and sustainability: Azospirillum as a model rhizotroph. In Rhizotrophs: Plant Growth Promotion to Bioremediation; Springer: Berlin/Heidelberg, Germany, 2017; pp. 15–37.
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971.
- Billah, M.; Khan, M.; Bano, A.; Hassan, T.U.; Munir, A.; Gurmani, A.R. Phosphorus and phosphate solubilizing bacteria: Keys for sustainable agriculture. Geomicrobiol. J. 2019, 36, 904–916.
- Garcia, C.L.; Dattamudi, S.; Chanda, S.; Jayachandran, K. Effect of salinity stress and microbial inoculations on glomalin and plant growth parameters of snap bean (Phaseolus vulgaris). Agronomy 2019, 9, 545.
- Alkurtany, A.; Ali, S.; Mahdi, W. The efficiency of prepared biofertilizer from local isolate of Bradyrhizobium sp on growth and yield of mungbean plant. Iraqi J. Agric. Sci. 2018, 49.
- Das, K.; Prasanna, R.; Saxena, A.K. Rhizobia: A potential biocontrol agent for soilborne fungal pathogens. Folia Microbiol. 2017, 62, 425–435.
- Mondal, M.; Skalicky, M.; Garai, S.; Hossain, A.; Sarkar, S.; Banerjee, H.; Kundu, R.; Brestic, M.; Barutcular, C.; Erman, M. Supplementing nitrogen in combination with rhizobium inoculation and soil mulch in peanut (Arachis hypogaea L.) production system: Part II. Effect on phenology, growth, yield attributes, pod quality, profitability and nitrogen use efficiency. Agronomy 2020, 10, 1513.
- Igiehon, N.O.; Babalola, O.O.; Aremu, B.R. Genomic insights into plant growth promoting rhizobia capable of enhancing soybean germination under drought stress. BMC Microbiol. 2019, 19, 159.
- Ali, M.A.; Ilyas, F.; Arshad, M.; Hussain, S.; Iqbal, M.; Ahmad, S.; Saboor, A.; Mustafa, G.; Ahmed, N. Microbial inoculation of seeds for better plant growth and productivity. In Priming and Pretreatment of Seeds and Seedlings; Springer: Berlin/Heidelberg, Germany, 2019; pp. 523–550.
- Rahman, M.; Sabir, A.A.; Mukta, J.A.; Khan, M.M.A.; Mohi-Ud-Din, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Plant probiotic bacteria Bacillus and Paraburkholderia improve growth, yield and content of antioxidants in strawberry fruit. Sci. Rep. 2018, 8, 1–11.
- Ahmed, B.; Zaidi, A.; Khan, M.S.; Rizvi, A.; Saif, S.; Shahid, M. Perspectives of plant growth promoting rhizobacteria in growth enhancement and sustainable production of tomato. In Microbial Strategies for Vegetablep Production; Springer: Berlin/Heidelberg, Germany, 2017; pp. 125–149.
- Gopalakrishnan, S.; Srinivas, V.; Samineni, S. Nitrogen fixation, plant growth and yield enhancements by diazotrophic growth-promoting bacteria in two cultivars of chickpea (Cicer arietinum L.). Biocatal. Agric. Biotechnol. 2017, 11, 116–123.
- Curá, J.A.; Franz, D.R.; Filosofía, J.E.; Balestrasse, K.B.; Burgueño, L.E. Inoculation with Azospirillum sp. and Herbaspirillum sp. bacteria increases the tolerance of maize to drought stress. Microorganisms 2017, 5, 41.
- Kandel, S.L.; Firrincieli, A.; Joubert, P.M.; Okubara, P.A.; Leston, N.D.; McGeorge, K.M.; Mugnozza, G.S.; Harfouche, A.; Kim, S.-H.; Doty, S.L. An in vitro study of bio-control and plant growth promotion potential of Salicaceae endophytes. Front. Microbiol. 2017, 8, 386.
- Ávila, J.S.; Ferreira, J.S.; Santos, J.S.; Rocha, P.A.d.; Baldani, V.L. Green manure, seed inoculation with Herbaspirillum seropedicae and nitrogen fertilization on maize yield. Rev. Bras. Eng. Agrícola Ambient. 2020, 24, 590–595.
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects a review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911.
- Sangeeth, K.; Bhai, R.S.; Srinivasan, V. Paenibacillus glucanolyticus, a promising potassium solubilizing bacterium isolated from black pepper (Piper nigrum L.) rhizosphere. J. Spices Arom. Crops 2012, 21, 118–124.
- Dias, M.P.; Bastos, M.S.; Xavier, V.B.; Cassel, E.; Astarita, L.V.; Santarém, E.R. Plant growth and resistance promoted by Streptomyces spp. in tomato. Plant Physiol. Biochem. 2017, 118, 479–493.
- Ghosh, R.; Mandal, N.C. Use of plant growth–promoting Burkholderia species with rock phosphate–solubilizing potential toward crop improvement. In Microbial Services in Restoration Ecology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 139–156.
- Kumar, H.; Dubey, R.; Maheshwari, D. Seed-coating fenugreek with Burkholderia rhizobacteria enhances yield in field trials and can combat Fusarium wilt. Rhizosphere 2017, 3, 92–99.
- Xu, X.; Xu, M.; Zhao, Q.; Xia, Y.; Chen, C.; Shen, Z. Complete genome sequence of Cd (II)-resistant Arthrobacter sp. PGP41, a plant growth-promoting bacterium with potential in microbe-assisted phytoremediation. Curr. Microbiol. 2018, 75, 1231–1239.
- Altuntaş, Ö. A comparative study on the effects of different conventional, organic and bio-fertilizers on broccoli yield and quality. Appl. Ecol. Environ. Res. 2018, 16, 1595–1608.
- Breitkreuz, C.; Buscot, F.; Tarkka, M.; Reitz, T. Shifts between and among populations of wheat rhizosphere Pseudomonas, Streptomyces and Phyllobacterium suggest consistent phosphate mobilization at different wheat growth stages under abiotic stress. Front. Microbiol. 2020, 10, 3109.
- Shinde, K.S.; Borkar, S. Seed bacterialization induced proline content in Sorghum bicolor crop under severe drought condition. Int. J. Chem. Stud. 2018, 6, 1191–1194.
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20.
- Reyes-Castillo, A.; Gerding, M.; Oyarzúa, P.; Zagal, E.; Gerding, J.; Fischer, S. Plant growth-promoting rhizobacteria able to improve NPK availability: Selection, identification and effects on tomato growth. Chilean J. Agric. Res. 2019, 79, 473–485.
- Patel, J.K.; Archana, G. Diverse culturable diazotrophic endophytic bacteria from Poaceae plants show cross-colonization and plant growth promotion in wheat. Plant Soil 2017, 417, 99–116.
- Ansari, M.H.; Hashemabadi, D.; Kaviani, B. Effect of Cattle Manure and Sulphur on Yield and Oil Composition of Pumpkin (Cucurbita pepo var. Styriaca) Inoculated with Thiobacillus thiooxidans in Calcareous Soil. Comm. Soil Sci. Plant Anal. 2017, 48, 2103–2118.
- Macedo-Raygoza, G.M.; Valdez-Salas, B.; Prado, F.M.; Prieto, K.R.; Yamaguchi, L.F.; Kato, M.J.; Canto-Canché, B.B.; Carrillo-Beltrán, M.; Di Mascio, P.; White, J.F. Enterobacter cloacae, an endophyte that establishes a nutrient-transfer symbiosis with banana plants and protects against the black Sigatoka pathogen. Front. Microbiol. 2019, 10, 804.
- Verma, P.; Agrawal, N.; Shahi, S.K. Effect of rhizobacterial strain Enterobacter cloacae strain pglo9 on potato plant growth and yield. Plant Arch. 2018, 18, 2528–2532.
- Paiter, A.; Freitas, G.; Pinto, L.; Hass, L.; Barreiros, M.; Oliveira, A.; Grange, L. IAA production and phosphate solubilization performed by native rhizobacteria in western Paraná. Agron. Sci. Biotechnol. 2019, 5, 70.
- Sagar, A.; Thomas, G.; Rai, S.; Mishra, R.K.; Ramteke, P.W. Enhancement of growth and yield parameters of wheat variety AAI-W6 by an organic farm isolate of plant growth promoting Erwinia Species (KP226572). Int. J. Agric. Environ. Biotechnol. 2018, 11, 159–171.
- Hernández-Montiel, L.G.; Chiquito Contreras, C.J.; Murillo Amador, B.; Vidal Hernández, L.; Quiñones Aguilar, E.E.; Chiquito Contreras, R.G. Efficiency of two inoculation methods of Pseudomonas putida on growth and yield of tomato plants. J. Soil Sci. Plant Nutr. 2017, 17, 1003–1012.
- Sattar, A.; Naveed, M.; Ali, M.; Zahir, Z.A.; Nadeem, S.M.; Yaseen, M.; Meena, V.S.; Farooq, M.; Singh, R.; Rahman, M. Perspectives of potassium solubilizing microbes in sustainable food production system: A review. Appl. Soil Ecol. 2019, 133, 146–159.
- Zhao, Y.; Zhang, M.; Yang, W.; Di, H.J.; Ma, L.; Liu, W.; Li, B. Effects of microbial inoculants on phosphorus and potassium availability, bacterial community composition, and chili pepper growth in a calcareous soil: A greenhouse study. J. Soils Sed. 2019, 19, 3597–3607.
- Pandey, D.; Kehri, H.K.; Zoomi, I.; Akhtar, O.; Singh, A.K. Mycorrhizal fungi: Biodiversity, ecological significance, and industrial applications. In Recent Advancement in White Biotechnology through Fungi; Springer: Berlin/Heidelberg, Germany, 2019; pp. 181–199.
- Bhale, U.; Bansode, S.; Singh, S. Multifactorial role of arbuscular mycorrhizae in agroecosystem. In Fungi and Their Role in Sustainable Development: Current Perspectives; Springer: Berlin/Heidelberg, Germany, 2018; pp. 205–220.
- Bhat, R.A.; Dervash, M.A.; Mehmood, M.A.; Skinder, B.M.; Rashid, A.; Bhat, J.I.A.; Singh, D.V.; Lone, R. Mycorrhizae: A sustainable industry for plant and soil environment. In Mycorrhiza-Nutrient Uptake, Biocontrol, Ecorestoration; Springer: Berlin/Heidelberg, Germany, 2017; pp. 473–502.
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ahmed, N.; Ashraf, M.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068.
- El Maaloum, S.; Elabed, A.; Alaoui-Talibi, Z.E.; Meddich, A.; Filali-Maltouf, A.; Douira, A.; Ibnsouda-Koraichi, S.; Amir, S.; El Modafar, C. Effect of arbuscular mycorrhizal fungi and phosphate-solubilizing bacteria consortia associated with phospho-compost on phosphorus solubilization and growth of tomato seedlings (Solanum lycopersicum L.). Commun. Soil Sci. Plant Analy. 2020, 51, 622–634.
- Xu, H.; Shao, H.; Lu, Y. Arbuscular mycorrhiza fungi and related soil microbial activity drive carbon mineralization in the maize rhizosphere. Ecotoxicol. Environ. Saf. 2019, 182, 109476.
- Chiomento, J.L.T.; Stürmer, S.L.; Carrenho, R.; da Costa, R.C.; Scheffer-Basso, S.M.; Antunes, L.E.C.; Nienow, A.A.; Calvete, E.O. Composition of arbuscular mycorrhizal fungi communities signals generalist species in soils cultivated with strawberry. Sci. Horticul. 2019, 253, 286–294.
- Tohidi Moghadam, H.; Heydarian, A.; Donath, T.; Sohrabi, M. Study of effect of arbuscular mycorrhiza (Glomus intraradices) fungus on wheat under nickel stress. Agron. Res. 2018, 16, 1660–1667.
- Krishnamoorthy, R.; Kim, K.; Subramanian, P.; Senthilkumar, M.; Anandham, R.; Sa, T. Arbuscular mycorrhizal fungi and associated bacteria isolated from salt-affected soil enhances the tolerance of maize to salinity in coastal reclamation soil. Agric. Ecosys. Environ. 2016, 231, 233–239.
- Boyer, L.R.; Brain, P.; Xu, X.-M.; Jeffries, P. Inoculation of drought-stressed strawberry with a mixed inoculum of two arbuscular mycorrhizal fungi: Effects on population dynamics of fungal species in roots and consequential plant tolerance to water deficiency. Mycorrhiza 2015, 25, 215–227.
- Mora-Romero, G.; Cervantes-Gámez, R.; Galindo-Flores, H.; González-Ortíz, M.; Félix-Gastélum, R.; Maldonado-Mendoza, I.E.; Pérez, R.S.; León-Félix, J.; Martínez-Valenzuela, M.; Lopez-Meyer, M. Mycorrhiza-induced protection against pathogens is both genotype-specific and graft-transmissible. Symbiosis 2015, 66, 55–64.
- Tognon, G.B.; Sanmartín, C.; Alcolea, V.; Cuquel, F.L.; Goicoechea, N. Mycorrhizal inoculation and/or selenium application affect post-harvest performance of snapdragon flowers. Plant Growth Regulat. 2016, 78, 389–400.
- Mota, R.M.A.; Fernández, A.d.J.R.; Trejo, J.B.; de la Cruz Elizondo, Y. Inoculación de hongos solubilizadores de fósforo y micorrizas arbusculares en plantas de jitomate. Rev. Mex. Cienc. Agrícolas 2019, 10, 1747–1757.
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 1–16.
- Saha, B.; Saha, S.; Roy, P.D.; Padhan, D.; Pati, S.; Hazra, G.C. Microbial transformation of sulphur: An approach to combat the sulphur deficiencies in agricultural soils. In Role of Rhizospheric Microbes in Soil; Springer: Berlin/Heidelberg, Germany, 2018; pp. 77–97.
- Pourbabaee, A.A.; Koohbori Dinekaboodi, S.; Seyed Hosseini, H.M.; Alikhani, H.A.; Emami, S. Potential application of selected sulphur-oxidizing bacteria and different sources of sulphur in plant growth promotion under different moisture conditions. Commun. Soil Sci. Plant Analy. 2020, 51, 735–745.
- Hejazirad, P.; Gholami, A.; Pirdashty, H.; Abbasiyan, A. Evaluation of Thiobacillus bacteria and mycorrhizal symbiosis on yield and yield components of garlic (Allium sativum) at different levels of sulphur. Agroecology 2017, 9, 76–87.
- da Silva Júnior, S.; Stamford, N.P.; Oliveira, W.S.; Silva, E.V.N.; de Rosalia e Silva Santos, C.E.; de Freitas, A.D.S.; da Silva, V.S.G. Microbial biofertilizer increases nutrient uptake on grape (Vitis labrusca L.) grown in an alkaline soil reclaimed by sulphur and’Acidithiobacillus’. Aust. J. Crop Sci. 2018, 12, 1695.
- Mącik, M.; Gryta, A.; Frąc, M. Biofertilizers in agriculture: An overview on concepts, strategies and effects on soil microorganisms. Adv. Agron. 2020, 160, 31.
- Dubey, R.; Gupta, D.K.; Sharma, G.K. Chemical stress on plants. In New Frontiers in Stress Management for Durable Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; pp. 101–128.
- Rajput, V.; Minkina, T.; Sushkova, S.; Behal, A.; Maksimov, A.; Blicharska, E.; Ghazaryan, K.; Movsesyan, H.; Barsova, N. ZnO and CuO nanoparticles: A threat to soil organisms, plants, and human health. Environ. Geochem. Health 2020, 42, 147–158.
- Ijaz, M.; Ali, Q.; Ashraf, S.; Kamran, M.; Rehman, A. Development of future bioformulations for sustainable agriculture. In Microbiome in Plant Health and Disease; Springer: Berlin/Heidelberg, Germany, 2019; pp. 421–446.
- Hussain, A.; Zahir, Z.A.; Ditta, A.; Tahir, M.U.; Ahmad, M.; Mumtaz, M.Z.; Hayat, K.; Hussain, S. Production and implication of bio-activated organic fertilizer enriched with zinc-solubilizing bacteria to boost up maize (Zea mays L.) production and biofortification under two cropping seasons. Agronomy 2020, 10, 39.
- Mumtaz, M.Z.; Ahmad, M.; Jamil, M.; Hussain, T. Zinc solubilizing Bacillus spp. potential candidates for biofortification in maize. Microbiol. Res. 2017, 202, 51–60.
- Naz, I.; Ahmad, H.; Khokhar, S.N.; Khan, K.; Shah, A.H. Impact of zinc solubilizing bacteria on zinc contents of wheat. American Eurasian J. Agric. Environ. Sci. 2016, 16, 449–454.
- Bhatt, K.; Maheshwari, D.K. Zinc solubilizing bacteria (Bacillus megaterium) with multifarious plant growth promoting activities alleviates growth in Capsicum annuum L. 3 Biotech 2020, 10, 36.
- Alori, E.T.; Dare, M.O.; Babalola, O.O. Microbial inoculants for soil quality and plant health. In Sustainable Agriculture Reviews; Springer: Berlin/Heidelberg, Germany, 2017; pp. 281–307.
- Pathak, D.; Kumar, M. Microbial inoculants as biofertilizers and biopesticides. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: Berlin/Heidelberg, Germany, 2016; pp. 197–209.
- Verma, D.K.; Pandey, A.K.; Mohapatra, B.; Srivastava, S.; Kumar, V.; Talukdar, D.; Yulianto, R.; Zuan, A.; Jobanputra, A.H.; Asthir, B. Plant growth-promoting rhizobacteria: An eco-friendly approach for sustainable agriculture and improved crop production. In Microbiology for Sustainable Agriculture, Soil Health, and Environmental Protection; Apple Academic Press: Canada, 2019; pp. 3–80.
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835.
- Brahmaprakash, G.; Sahu, P.K.; Lavanya, G.; Nair, S.S.; Gangaraddi, V.K.; Gupta, A. Microbial functions of the rhizosphere. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: Singerpore, 2017; pp. 177–210.
- Khalid, M.; Hassani, D.; Bilal, M.; Asad, F.; Huang, D. Influence of bio-fertilizer containing beneficial fungi and rhizospheric bacteria on health promoting compounds and antioxidant activity of Spinacia oleracea L. Bot. Stud. 2017, 58, 35.
- Arora, M.; Saxena, P.; Abdin, M.; Varma, A. Interaction between Piriformospora indica and Azotobacter chroococcum governs better plant physiological and biochemical parameters in Artemisia annua L. plants grown under in vitro conditions. Symbiosis 2018, 75, 103–112.
- Kapoor, R.; Singh, N. Arbuscular mycorrhiza and reactive oxygen species. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Springer: Berlin/Heidelberg, Germany, 2017; pp. 225–243.
- Hassen, A.I.; Bopape, F.; Sanger, L. Microbial inoculants as agents of growth promotion and abiotic stress tolerance in plants. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: Berlin/Heidelberg, Germany, 2016; pp. 23–36.
- Alori, E.T.; Babalola, O.O. Microbial inoculants for improving crop quality and human health in Africa. Front. Microbiol. 2018, 9, 2213.
- Dicko, A.H.; Babana, A.H.; Kassogué, A.; Fané, R.; Nantoumé, D.; Ouattara, D.; Maiga, K.; Dao, S. A Malian native plant growth promoting Actinomycetes based biofertilizer improves maize growth and yield. Symbiosis 2018, 75, 267–275.
- Win, K.T.; Okazaki, K.; Ookawa, T.; Yokoyama, T.; Ohwaki, Y. Influence of rice-husk biochar and Bacillus pumilus strain TUAT-1 on yield, biomass production, and nutrient uptake in two forage rice genotypes. PLoS ONE 2019, 14, e0220236.
- Fathi, A. Effect of phosphate solubilization microorganisms and plant growth promoting rhizobacteria on yield and yield components of corn. Sci. Agric. 2017, 18, 66–69.
- Yu, C.; Huang, X.; Chen, H.; Godfray, H.C.J.; Wright, J.S.; Hall, J.W.; Gong, P.; Ni, S.; Qiao, S.; Huang, G. Managing nitrogen to restore water quality in China. Nature 2019, 567, 516–520.
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O. Plant health: Feedback effect of root exudates-rhizobiome interactions. Appl. Microbiol. Biotechnol. 2019, 103, 1155–1166.
- Soumare, A.; Boubekri, K.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. From isolation of phosphate solubilizing microbes to their formulation and use as biofertilizers: Status and needs. Front. Bioengin. Biotechnol. 2019, 7, 425.
- Berninger, T.; González López, Ó.; Bejarano, A.; Preininger, C.; Sessitsch, A. Maintenance and assessment of cell viability in formulation of non-sporulating bacterial inoculants. Microb. Biotechnol. 2018, 11, 277–301.
- Choube, G.; Karadbhajne, D.V.; Johi, D.Y.; Jambhekar, D.H.; Dhargave, T. A pilot scale process for the production of high shelf life multi-functional liquid bio-fertilizer. Int. J. Biotechnol. Res. 2018, 8, 1–10.
- Lesueur, D.; Deaker, R.; Herrmann, L.; Bräu, L.; Jansa, J. The production and potential of biofertilizers to improve crop yields. In Bioformulations: For Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 71–92.
- Datta, A.; Ullah, H.; Ferdous, Z. Utilization of by-products from food processing as biofertilizers and biopesticides. In Food Processing By-Products and their Utilization; Asian Institute of Technology: Pathumthani, Thailand, 2017; pp. 175–193.
- Vassilev, N.; Vassileva, M.; Lopez, A.; Martos, V.; Reyes, A.; Maksimovic, I.; Eichler-Löbermann, B.; Malusa, E. Unexploited potential of some biotechnological techniques for biofertilizer production and formulation. Appl. Microbiol. Biotechnol. 2015, 99, 4983–4996.
- Paliya, S.; Mandpe, A.; Kumar, S.; Kumar, M.S. Enhanced nodulation and higher germination using sludge ash as a carrier for biofertilizer production. J. Environ. Manag. 2019, 250, 109523.
- Bharathi, R.; Vivekananthan, R.; Harish, S.; Ramanathan, A.; Samiyappan, R. Rhizobacteria-based bio-formulations for the management of fruit rot infection in chillies. Crop Prot. 2004, 23, 835–843.
- Saravanakumar, G.; Choi, K.Y.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Park, K. Hydrotropic hyaluronic acid conjugates: Synthesis, characterization, and implications as a carrier of paclitaxel. Int. J. Pharm. 2010, 394, 154–161.
- Herrmann, L.; Lesueur, D. Challenges of formulation and quality of biofertilizers for successful inoculation. Appl. Microbiol. Biotechnol. 2013, 97, 8859–8873.
- Thirumal, G.; Reddy, R.S.; Triveni, S.; Damodarachari, K.; Bhavya, K. Evaluate the shelf life of Rhizobium carrier based biofertilizer stored at different temperatures at different intervals. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 753–759.
- Roychowdhury, D.; Paul, M.; Kumar Banerjee, S. Isolation identification and characterization of phosphate solubilizing bacteria from soil and the production of biofertilizer. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 808–815.
- Wang, H.-y.; Shen, L.; Zhai, L.-m.; Zhang, J.-z.; Ren, T.-z.; Fan, B.-q.; LIU, H.-b. Preparation and utilization of phosphate biofertilizers using agricultural waste. J. Integr. Agric. 2015, 14, 158–167.
- Hassan, T.U.; Bano, A. Biofertilizer: A novel formulation for improving wheat growth, physiology and yield. Pak. J. Bot. 2016, 48, 2233–2241.
- Rodrigues, K.; Rodrigues, B. Development of carrier based in vitro produced arbuscular mycorrhizal (AM) fungal inocula for organic agriculture. Annals Advan. Agric. Sci. 2017, 1, 26–37.
- Shravani, K. Evaluation of shelf life and quality of carrier and liquid based biofertilizers. Int. J. Microbiol. Res. 2019, 11, 1598–1601.
- Devi, T.S.; Gupta, S.; Kapoor, R. Arbuscular mycorrhizal fungi in alleviation of cold stress in plants. In Advancing Frontiers in Mycology & Mycotechnology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 435–455.
- Kumar, A.; Singh, R.; Adholeya, A. Biotechnological advancements in industrial production of arbuscular mycorrhizal fungi: Achievements, challenges, and future prospects. In Developments in Fungal Biology and Applied Mycology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 413–431.
- Gryndler, M.; Šmilauer, P.; Püschel, D.; Bukovská, P.; Hršelová, H.; Hujslová, M.; Gryndlerová, H.; Beskid, O.; Konvalinková, T.; Jansa, J. Appropriate nonmycorrhizal controls in arbuscular mycorrhiza research: A microbiome perspective. Mycorrhiza 2018, 28, 435–450.
- Mishra, S.; Keswani, C.; Abhilash, P.; Fraceto, L.F.; Singh, H.B. Integrated approach of agri-nanotechnology: Challenges and future trends. Front. Plant Sci. 2017, 8, 471.
- Veronica, N.; Guru, T.; Thatikunta, R.; Reddy, S.N. Role of Nano fertilizers in agricultural farming. Int. J. Environ. Sci. Technol. 2015, 1, 1–3.
- Kumari, R.; Singh, D.P. Nano-biofertilizer: An emerging eco-friendly approach for sustainable agriculture. In Proceedings of the National Academy of Sciences, India Section B: Biological Sciences; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–9.
