Salvia divinorum Epling and Játiva is a psychoactive Mexican mint used for centuries by Mazatec Indian shamans or curanderos of north-eastern Oaxaca, Mexico, for divinatory and religious purposes and physical healing.
- hallucinogens
- herbal highs
- opioid receptors
- pharmacodynamics
1. Introduction
Salvia divinorum Epling and Játiva is a psychoactive Mexican mint used for centuries by Mazatec Indian shamans or curanderos of north-eastern Oaxaca, Mexico, for divinatory and religious purposes and physical healing [1,2][1][2]. Common vernacular names include Sally D, Magic Mint, Diviner’s Sage, Mystic Sage, Purple Sticky, Lady Salvia, or just Salvia. As Mazatecans believe that this herb is the reincarnation of the Virgin Mary, the term Maria Pastora is also used [3,4][3][4]. The first botanical description of the plant dates from 1963 [5]. However, it was only in the 1990s that S. divinorum was brought to the United States (US) and its psychoactive properties were recognised [2].
The main bioactive compound of this plant is salvinorin A, the first non-nitrogenous diterpenoid with psychoactive properties that was described, which was first isolated from the leaves of S. divinorum in 1982 [6]. It is one of the most potent natural hallucinogens known to date [7], with 200 µg being sufficient to trigger biological effects in humans after smoking [2]. From a pharmacological and chemical point of view, salvinorin A is quite unique due to two main features: (i) it is the only known non-alkaloidal hallucinogen, and (ii) it is the first naturally occurring non-nitrogenous compound that acts as an efficacious and potent κ-opioid receptor (KOP)-selective agonist [8,9,10,11][8][9][10][11]. Although not having affinity for the 5-HT2A receptors known to mediate the hallucinogenic properties of the classic psychedelics, such as lysergic acid diethylamide (LSD), psilocybin [12], mescaline [13], and N,N-dimethyltryptamine (DMT) [14], salvinorin A induces similar psychoactive effects, including perceptual distortions, alterations in affect, behaviour, and cognition, and out-of-body experiences, with such effects being intense and short-lived [3,15][3][15]. The consumption of this substance has been associated with minimal or no health risks, thus being conceded as a safe hallucinogen [3].
The recreational use of this novel psychoactive drug has increased within the last 20 years, both in the US and Europe [16], mainly by adolescents and young adults [4]. In fact, it gained substantial popularity as a substitute for other common psychoactive substances, namely, cannabis derivatives and LSD [17], and through a search of YouTube videos it is possible to verify that S. divinorum and salvinorin A are becoming trendy topics [18,19][18][19]. Further fuelling this growing recreational use, leaf preparations of S. divinorum can be easily found and purchased over the Internet and in smartshops [3,20][3][20]. Seeds to be cultivated by the consumer; leaves that can be chewed, brewed, or dried for smoking; and liquid extracts used as a drink or smoked in water pipes are the forms under which the plant is normally sold [21]. Leaf materials can be sporadically found impregnated/fortified with salvinorin A extracts, enhancing the concentration of the substance and resulting in increased potency of the sold products (1–80x) [22]. Other factors contributing to its increased use are that S. divinorum enjoys legality in some countries, is apparently safe, and lacks detectability by commonly used drug screening tests [4]. The growing public representation of S. divinorum led to an increased mindfulness of its hallucinogenic properties, and apprehension about potential harmful outcomes of its use [23]. In fact, although S. divinorum is still an unregulated psychoactive plant in many countries, the United Nations Office on Drugs and Crime (UNODC) considered it a new psychoactive drug of concern [24].
Potential medicinal benefits of S. divinorum have long been recognised, mainly due to its use in folk medicine by the Mazatecans for the treatment of inflammatory disorders, rheumatism, headache, abdominal swelling, and diarrhoea [1,25][1][25]. Additional ethnomedical surveys also indicate its use for insect bites, eczema, candidiasis, and menstrual cramps [26]. An intricate and complex pharmacology and mechanism of action of this hallucinatory plant may be implicated in these several medicinal applications.
2. Plant Botany and Chemical Characterisation of S. divinorum
S. divinorum is a perennial herb, endemic to the forest ravines of Sierra Madre Oriental of Oaxaca, Mexico [27]. Large green leaves and flowers with purple calyces and white corollas can be seen in S. divinorum plants (Figure 1) [3]. A maximum height of 1.5 m can be reached by these plants, and the leaves can be 10–25 cm long and 5–10 cm wide [5]. Cultivation of S. divinorum is usually accomplished at altitudes ranging between 750 and 1500 m, under hot, moist, humid subtropical environmental conditions [28]. S. divinorum is mainly widespread in Mexico and South American countries. Although the plant produces viable seeds, this is not frequent [5], with propagation being accomplished through vegetative reproduction.

Figure 1. Salvia divinorum plant (A), the characteristic flower (B), and dry leaves (C).
Salvia spp. are known to be prolific producers of terpenes and terpenoids, particularly diterpenes, with the most common class of phytochemicals being reported in the genus [29]. Besides the main psychoactive constituent salvinorin A, other neoclerodane diterpenes have been isolated from the leaves of S. divinorum, which include salvinorins B–J, divinatorins A–F, salvidivins A–D, and salvinicins A and B [30,31,32,33,34,35][30][31][32][33][34][35]. Salvinorin A accumulates in large amounts on the glandular trichomes of the abaxial side of leaves [36]. Salvinorins were not observed in the roots, internal stem tissue, cotyledons, or corolla [36].
Concentrations of salvinorin A in the leaves of a single specimen can be extremely consistent over time [37]. However, different S. divinorum plants, even when genetically undistinguishable, can present considerable distinct concentrations in the leaves, varying from 0.89 to 7.8 mg/g of dried leaves. Table 1 displays salvinorin content in samples locally harvested or commercially obtained. The high variability in the amounts found might depend on the freshness of the leaves [38], geographic origin of the plant, cultivation technique [17[17][39],39], and adulteration of the traded products [40].
Table 1. Concentrations of salvinorin A and salvinorin B reported in different S. divinorum samples.
| Sample | Salvinorin A (mg/g) |
Salvinorin B (mg/g) |
Reference | |||
|---|---|---|---|---|---|---|
| Leaves from private collections | 1 | and endemic populations of Oaxaca | 2 | 0.89–3.70 | - | [41] |
| Leaves from plants endemic to Sierra Mazatecan | 7.6 | 4.20 | [39] | |||
| Leaves from Hawaiian plants | 7.8 | 10.4 | [39] | |||
| Leaves and extracts purchased on the Internet (1–20×) | 0.126–1.137 | - | [40] | |||
| Dried leaves and concentrated extract products purchased on Japanese drug market (1×) | 3.2–5.0 | 0.10–0.17 | [22] | |||
| Ground young leaves of plants purchased from the Vancouver Seed Bank | 0.9 | [42] |
1 Wasson and Hofmann clones and “Palatable” clones. 2 Cerro Rabón collection and Huatla de Jimenez collection. (-): Not mentioned.
3. Biosynthesis, Structure, and Physicochemical Properties of Salvinorin A
The biosynthesis of salvinorin A occurs through the 2-C-methyl-erythritol 4-phosphate (MEP) pathway, involving class I and class II diterpene synthase (diTPS)-mediated reactions [42]. The precursor of this biosynthetic pathway was shown to be geranylgeranyl diphosphate (GGPP), which undergoes cycloisomerisation mediated by class II diTPS. The product of this enzymatic reaction is (–)-kolavenyl diphosphate, which is subsequently dephosphorylated by class I diTPS, rendering (–)-kolavenol. Regio- and stereoselective reactions further enable the production of a variety of intermediates and precursors of salvinorin A [42].
The total chemical synthesis of salvinorin A was first accomplished in 2007 through an asymmetric route [43] that featured a transannular Michael reaction cascade from a macrocyclic lactone, encompassing 29 reaction steps. The most recent advances in the topic were made by Wang and Metz [44], who developed a synthetic method involving only 18 reactions. Using two highly diastereoselective intramolecular Diels–Alder reactions as crucial transformations, salvinorin A was obtained from 3-furaldehyde. A comprehensive review on the total synthesis of salvinorin A was recently provided by Hill et al. [45].
Unlike the other naturally occurring hallucinogens, salvinorin A (Figure 2) is a terpenoid that does not have nitrogen atoms in its molecular formulae (C23H28O8).
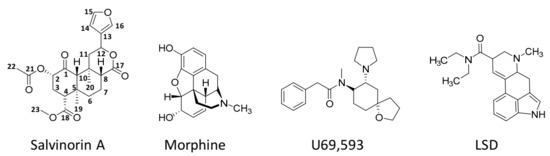
Figure 2. Chemical structures of salvinorin A, morphine (a well-known opioid), U69,593 (a potent and selective synthetic κ-opioid receptor agonist), and lysergic acid diethylamide (LSD, a recognised hallucinogen).
Pure salvinorin A is a colourless crystal with a high melting point, ranging from 238 to 240 °C. Salvinorin A is also distinguished from the majority of psychoactives as it is highly lipophilic [19], presenting limited solubility in water, with a logP (octanol/water) of 2.5 [46]. Soluble salts of salvinorin A cannot be formed due to the nonexistence of an ionisable functional group [47]. Salvinorin A has also low solubility in conventional safe vehicles applied in animal studies, with DMSO being commonly used instead [48]. It is worth noting that the high lipophilicity of salvinorin A enables passage through biological membranes, including the blood–brain barrier (BBB) [49].
References
- Valdés, L.J., 3rd; Díaz, J.L.; Paul, A.G. Ethnopharmacology of ska María Pastora (Salvia divinorum, Epling and Játiva-M.). J. Ethnopharmacol. 1983, 7, 287–312.
- Siebert, D.J. Salvia divinorum and salvinorin A: New pharmacologic findings. J. Ethnopharmacol. 1994, 43, 53–56.
- Ahern, N.R.; Greenberg, C.S. Psychoactive herb use and youth: A closer look at salvia divinorum. J. Psychosoc. Nurs. Ment. Health Serv. 2011, 49, 16–19.
- Babu, K.M.; McCurdy, C.R.; Boyer, E.W. Opioid receptors and legal highs: Salvia divinorum and Kratom. Clin. Toxicol. 2008, 46, 146–152.
- Reisfield, S.A. The botany of Salvia divinorum (Labiatae). SIDA 1993, 15, 349–366.
- Ortega, A.; Blount, J.F.; Manchand, P.S. Salvinorin, a new trans-neoclerodane diterpene from Salvia divinorum. J. Chem. Soc. Perkin Trans. I 1982, 1, 2505–2508.
- Valdés, L.J. 3rd. Salvia divinorum and the unique diterpene hallucinogen, Salvinorin (divinorin) A. J. Psychoact. Drugs 1994, 26, 277–283.
- Sheffler, D.J.; Roth, B.L. Salvinorin A: The “magic mint” hallucinogen finds a molecular target in the kappa opioid receptor. Trends Pharmacol. Sci. 2003, 24, 107–109.
- Yan, F.; Roth, B.L. Salvinorin A: A novel and highly selective kappa-opioid receptor agonist. Life Sci. 2004, 75, 2615–2619.
- Roth, B.L.; Baner, K.; Westkaemper, R.; Siebert, D.; Rice, K.C.; Steinberg, S.; Ernsberger, P.; Rothman, R.B. Salvinorin A: A potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc. Natl. Acad. Sci. USA 2002, 99, 11934–11939.
- Chavkin, C.; Sud, S.; Jin, W.; Stewart, J.; Zjawiony, J.K.; Siebert, D.J.; Toth, B.A.; Hufeisen, S.J.; Roth, B.L. Salvinorin A, an active component of the hallucinogenic sage salvia divinorum is a highly efficacious kappa-opioid receptor agonist: Structural and functional considerations. J. Pharmacol. Exp. Ther. 2004, 308, 1197–1203.
- Dinis-Oliveira, R.J. Metabolism of psilocybin and psilocin: Clinical and forensic toxicological relevance. Drug Metab. Rev. 2017, 49, 84–91.
- Dinis-Oliveira, R.J.; Pereira, C.L.; Da Silva, D.D. Pharmacokinetic and Pharmacodynamic Aspects of Peyote and Mescaline: Clinical and Forensic Repercussions. Curr. Mol. Pharmacol. 2019, 12, 184–194.
- Brito-da-Costa, A.M.; Dias-da-Silva, D.; Gomes, N.G.M.; Dinis-Oliveira, R.J.; Madureira-Carvalho, Á. Toxicokinetics and Toxicodynamics of Ayahuasca Alkaloids N,N-Dimethyltryptamine (DMT), Harmine, Harmaline and Tetrahydroharmine: Clinical and Forensic Impact. Pharmaceuticals 2020, 13, 334.
- Baggott, M.J.; Erowid, E.; Erowid, F.; Galloway, G.P.; Mendelson, J. Use patterns and self-reported effects of Salvia divinorum: An internet-based survey. Drug Alcohol Depend. 2010, 111, 250–256.
- Wu, L.T.; Woody, G.E.; Yang, C.; Li, J.H.; Blazer, D.G. Recent national trends in Salvia divinorum use and substance-use disorders among recent and former Salvia divinorum users compared with nonusers. Subst. Abuse Rehabil. 2011, 2011, 53–68.
- Barnes, B.B.; Snow, N.H. Analysis of Salvinorin A in plants, water, and urine using solid-phase microextraction-comprehensive two-dimensional gas chromatography-time of flight mass spectrometry. J. Chromatogr. A 2012, 1226, 110–115.
- Casselman, I.; Heinrich, M. Novel use patterns of Salvia divinorum: Unobtrusive observation using YouTube™. J. Ethnopharmacol. 2011, 138, 662–667.
- Baker, L.E.; Panos, J.J.; Killinger, B.A.; Peet, M.M.; Bell, L.M.; Haliw, L.A.; Walker, S.L. Comparison of the discriminative stimulus effects of salvinorin A and its derivatives to U69,593 and U50,488 in rats. Psychopharmacology 2009, 203, 203–211.
- Prisinzano, T.E. Psychopharmacology of the hallucinogenic sage Salvia divinorum. Life Sci. 2005, 78, 527–531.
- Johnson, M.W.; MacLean, K.A.; Reissig, C.J.; Prisinzano, T.E.; Griffiths, R.R. Human psychopharmacology and dose-effects of salvinorin A, a kappa opioid agonist hallucinogen present in the plant Salvia divinorum. Drug Alcohol Depend. 2011, 115, 150–155.
- Tsujikawa, K.; Kuwayama, K.; Miyaguchi, H.; Kanamori, T.; Iwata, Y.T.; Yoshida, T.; Inoue, H. Determination of salvinorin A and salvinorin B in Salvia divinorum-related products circulated in Japan. Forensic Sci. Int. 2008, 180, 105–109.
- Sumnall, H.R.; Measham, F.; Brandt, S.D.; Cole, J.C. Salvia divinorum use and phenomenology: Results from an online survey. J. Psychopharmacol. 2011, 25, 1496–1507.
- Hammond, B.; Crean, C.; Levissianos, S.; Mermerci, D.; Tun Nay, S.; Otani, T.; Park, M.; Pazos, D.; Piñeros, K.; Umapornsakula, A.; et al. The Challenge of New Psychoactive Substances; UNODC Global SMART Programme: Vienna, Austria, 2013.
- Vortherms, T.A.; Roth, B.L. Salvinorin A: From natural product to human therapeutics. Mol. Interv. 2006, 6, 257–265.
- Maqueda, A.E. The use of Salvia divinorum from a Mazatec perspective. In Plant Medicines, Healing and Psychedelic Science; Labate, B.C., Cavnar, C., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 55–70.
- Akaberi, M.; Iranshahi, M.; Mehri, S. Molecular Signaling Pathways behind the Biological Effects of Salvia Species Diterpenes in Neuropharmacology and Cardiology. Phytother. Res. 2016, 30, 878–893.
- Braida, D.; Donzelli, A.; Martucci, R.; Capurro, V.; Sala, M. Learning and memory impairment induced by salvinorin A, the principal ingredient of Salvia divinorum, in wistar rats. Int. J. Toxicol. 2011, 30, 650–661.
- Wu, Y.B.; Ni, Z.Y.; Shi, Q.W.; Dong, M.; Kiyota, H.; Gu, Y.C.; Cong, B. Constituents from Salvia species and their biological activities. Chem. Rev. 2012, 112, 5967–6026.
- Cunningham, C.W.; Rothman, R.B.; Prisinzano, T.E. Neuropharmacology of the naturally occurring kappa-opioid hallucinogen salvinorin A. Pharmacol. Rev. 2011, 63, 316–347.
- Munro, T.A.; Rizzacasa, M.A. Salvinorins D-F, new neoclerodane diterpenoids from Salvia divinorum, and an improved method for the isolation of salvinorin A. J. Nat. Prod. 2003, 66, 703–705.
- Bigham, A.K.; Munro, T.A.; Rizzacasa, M.A.; Robins-Browne, R.M. Divinatorins A-C, new neoclerodane diterpenoids from the controlled sage Salvia divinorum. J. Nat. Prod. 2003, 66, 1242–1244.
- Harding, W.W.; Tidgewell, K.; Schmidt, M.; Shah, K.; Dersch, C.M.; Snyder, J.; Parrish, D.; Deschamps, J.R.; Rothman, R.B.; Prisinzano, T.E. Salvinicins A and B, new neoclerodane diterpenes from Salvia divinorum. Org. Lett. 2005, 7, 3017–3020.
- Lee, D.Y.; Ma, Z.; Liu-Chen, L.Y.; Wang, Y.; Chen, Y.; Carlezon, W.A., Jr.; Cohen, B. New neoclerodane diterpenoids isolated from the leaves of Salvia divinorum and their binding affinities for human kappa opioid receptors. Bioorg. Med. Chem. 2005, 13, 5635–5639.
- Casselman, I.; Nock, C.J.; Wohlmuth, H.; Weatherby, R.P.; Heinrich, M. From local to global-fifty years of research on Salvia divinorum. J. Ethnopharmacol. 2014, 151, 768–783.
- Siebert, D.J. Localization of salvinorin A and related compounds in glandular trichomes of the psychoactive sage, Salvia divinorum. Ann. Bot. 2004, 93, 763–771.
- Bertea, C.M.; Luciano, P.; Bossi, S.; Leoni, F.; Baiocchi, C.; Medana, C.; Azzolin, C.M.; Temporale, G.; Lombardozzi, M.A.; Maffei, M.E. PCR and PCR-RFLP of the 5S-rRNA-NTS region and salvinorin A analyses for the rapid and unequivocal determination of Salvia divinorum. Phytochemistry 2006, 67, 371–378.
- Grundmann, O.; Phipps, S.M.; Zadezensky, I.; Butterweck, V. Salvia divinorum and salvinorin A: An update on pharmacology and analytical methodology. Planta Med. 2007, 73, 1039–1046.
- Medana, C.; Massolino, C.; Pazzi, M.; Baiocchi, C. Determination of salvinorins and divinatorins in Salvia divinorum leaves by liquid chromatography/multistage mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 131–136.
- Wolowich, W.R.; Perkins, A.M.; Cienki, J.J. Analysis of the psychoactive terpenoid salvinorin A content in five Salvia divinorum herbal products. Pharmacotherapy 2006, 26, 1268–1272.
- Gruber, J.W.; Siebert, D.J.; Der Marderosian, A.H.; Hock, R.S. High performance liquid chromatographic quantification of salvinorin A from tissues of Salvia divinorum Epling & Játiva-M. Phytochem. Anal. 1999, 10, 22–25.
- Pelot, K.A.; Mitchell, R.; Kwon, M.; Hagelthorn, L.M.; Wardman, J.F.; Chiang, A.; Bohlmann, J.; Ro, D.K.; Zerbe, P. Biosynthesis of the psychotropic plant diterpene salvinorin A: Discovery and characterization of the Salvia divinorum clerodienyl diphosphate synthase. Plant J. 2017, 89, 885–897.
- Scheerer, J.R.; Lawrence, J.F.; Wang, G.C.; Evans, D.A. Asymmetric synthesis of salvinorin A, a potent kappa opioid receptor agonist. J. Am. Chem. Soc. 2007, 129, 8968–8969.
- Wang, Y.; Metz, P. Total Synthesis of the Neoclerodane Diterpene Salvinorin A via an Intramolecular Diels-Alder Strategy. Org. Lett. 2018, 20, 3418–3421.
- Hill, S.J.; Brion, A.; Shenvi, R.A. Chemical syntheses of the salvinorin chemotype of KOR agonist. Nat. Prod. Rep. 2020.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 128563, Salvinorin A. Available online: (accessed on 10 November 2020).
- Orton, E.; Liu, R. Salvinorin A: A Mini Review of Physical and Chemical Properties Affecting Its Translation from Research to Clinical Applications in Humans. Transl. Perioper Pain Med. 2014, 1, 9–11.
- Ansonoff, M.A.; Zhang, J.; Czyzyk, T.; Rothman, R.B.; Stewart, J.; Xu, H.; Zjwiony, J.; Siebert, D.J.; Yang, F.; Roth, B.L.; et al. Antinociceptive and hypothermic effects of Salvinorin A are abolished in a novel strain of kappa-opioid receptor-1 knockout mice. J. Pharmacol. Exp. Ther. 2006, 318, 641–648.
- Teksin, Z.S.; Lee, I.J.; Nemieboka, N.N.; Othman, A.A.; Upreti, V.V.; Hassan, H.E.; Syed, S.S.; Prisinzano, T.E.; Eddington, N.D. Evaluation of the transport, in vitro metabolism and pharmacokinetics of Salvinorin A, a potent hallucinogen. Eur. J. Pharm. Biopharm. 2009, 72, 471–477.
