Tendons are hypocellular and hypovascular tissues, and thus, their natural healing capacity is low. In this study, we sought to evaluate the efficacy of platelet-rich fibrin (PRF) to serve as a bioactive scaffold in promoting the healing of rabbit Achilles tendon injury. For in vitro study, the essence portion of PRF was determined through bioluminescent assay. Furthermore, we analyzed the time-sequential cytokines-release kinetics of PRF and evaluated their effects on tenocytes proliferation and tenogenic gene expressions. In animal study, the rabbit Achilles tendon defect was left untreated or implanted with normal/heat-denatured PRF scaffolds. Six weeks postoperatively, the specimens were evaluated through sonographic imaging and histological analysis. The results revealed significantly more activated platelets on bottom half of the PRF scaffold. Cytokine concentrations released from PRF could be detected from the first hour to six days. For the in vitro study, PRF enhanced cell viability and collagen I, collagen III, tenomodulin, and tenascin gene expression compared to the standard culture medium. For in vivo study, sonographic images revealed significantly better tendon healing in the PRF group in terms of tissue echogenicity and homogeneity. The histological analysis showed that the healing tissues in the PRF group had more organized collagen fiber, less vascularity, and minimal cartilage formation. In conclusion, bioactive PRF promotes in vitro tenocytes viability and tenogenic phenotypic differentiation. Administration of a PRF scaffold at the tendon defect promotes tissue healing as evidenced by imaging and histological outcomes.
- Implantation
- cytokine-delivery
- platelet-rich fibrin
- Achilles tendon
- healing
1. In ViResultros
In Vitro
1.1. Quantification of ATP Contents Released from Activated Platelets
Quantification of ATP Contents Released from Activated Platelets
When platelets are activated by thrombotic stimuli, their contained ATP is secreted extracellularly (Figure 1A) [1][24]. Therefore, the bioactivity of PRF was determined through determining local adenosine triphosphate (ATP) released from activated platelets. Figure 1B showed the reaction equation of firefly luciferase assay. The assay is based on luciferase’s requirement for ATP in producing light (emission maximum ~560 nm at pH 7.8). Figure 1C showed the acquired bioluminescence imaging of PRF sample observed under fluorescence optical microscope. The bioluminescence light can be detected across the entire PRF gel, and the image brightness was recorded and quantitated. According to the reaction equation, the light production by cell-surface-attached luciferase is continuous and linearly related to ATP concentration. Figure 1D showed the ATP concentrations of PRF samples derived from five different rabbits.
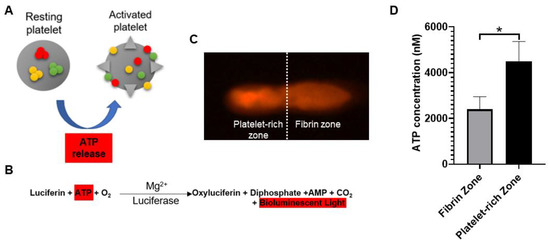
Figure 1. (A) Adenosine triphosphate (ATP) is released from dense granules during platelet activation. (B) Detection of activated platelets on platelet-rich fibrin (PRF) through bioluminescent assay. (C) PRF sample was observed under a fluorescence optical microscope. Based on bioluminescent intensity, the PRF could be equally divided into fibrin zone and platelet-rich zone. (D) The image brightness of bioluminescence was substituted into the ATP standard curve to determine the concentration of platelet-released ATP. The bars show the mean ± SD (n = 5) of each group. * p < 0.05.
1.2. Time-Sequential Cytokine Release
Time-Sequential Cytokine Release
Figure 2A–E showed the time-sequential release pattern of five different types of cytokine starting from 1 h until day 6. The results demonstrated that platelet-derived growth factor-AA (PDGF-AA), platelet-derived growth factor-BB (PDGF- BB) and fibroblast growth factor-2 (FGF-2) content released from PRF increased significantly over the time course of the study. For PDGF-AA and PDGF-BB, the cytokine concentrations peaked at 48 h and 72 h, respectively, with mean values of 473.12 ± 19.8 ng/L and 214.26 ± 5.82 ng/L (Figure 2A,B). For FGF-2, the cytokine concentration gradually increased and reached top at 72 h, with mean values of 2168.14 ± 83.5 ng/L (Figure 2C). In contrast, the transforming growth factor-β (TGF-β) concentration remained stable without much fluctuation with mean value of 52.6 ± 1.42 ng/L (Figure 2D). The level of insulin-like growth factor-1 (IGF-1) decreased after 6 h and could not be detected after 48 h (Figure 2E).
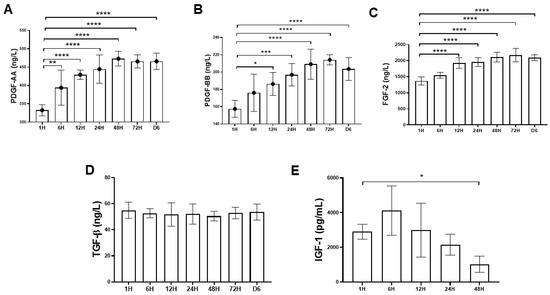
Figure 2. Time-sequential release profile of platelet-derived growth factor-AA (PDGF-AA), platelet-derived growth factor-BB (PDGF-BB), fibroblast growth factor-2 (FGF-2), transforming growth factor-β (TGF-β), and insulin-like growth factor-1 (IGF-1) from first hour to six days. The PDGF-AA, PDGF-BB, FGF-2, and TGF-β1 releases were sustained over six days. (A–C) The PDGF-AA, PDGF-BB, and FGF-2 releases were rapidly induced, and a maximum concentration was detected at 48 h and 72 h. (D) The TGF-β1 release was constant and sustained over six-day period. (E) The IGF-1 release decreased over time and could not be detected after 48 h. The bars show the mean ± SD fold change (n = 6) of each group. * p < 0.05; ** p< 0.01; *** p < 0.001; **** p < 0.0001.
1.3. Dose-Dependent Effects of PRF on Tenocytes Viability and Proliferation
Dose-Dependent Effects of PRF on Tenocytes Viability and Proliferation
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was used to evaluate the differential effects of two types of PRF-conditioned medium (PRFM), known as the fibrin zone PRFM and platelet-rich zone PRFM on tenocytes viability during nine-day cultivation. Figure 3A–C shows that significantly higher cell viability and proliferation were noted in tenocytes treated with platelet-rich zone PRFM compared to fibrin zone-PRFM, particularly on day 6 and day 9. On day 6, the 25% (1.21 + 0.06), 50% (1.52 ± 0.05), and 100% platelet-rich zone PRFM (1.66 ± 0.02) has a 1.2- to 1.5-fold increase in cell numbers compared to fibrin-zone-PRFM. On day 9, the potent effects of platelet-rich zone PRFM on cell viability remained comparable to its counterpart fibrin-zone PRFM (p < 0.001). The data clearly displayed the differential effects of two different PRFMs on cell viability, suggesting that platelet-rich zone-PRF may contain a higher amount of cytokines responsible for increased cell growth. Figure 3D,E confirms that increasing concentrations of PRFM did increase the cell viability of cultured tenocytes in a dose-dependent manner, both in fibrin zone PRFM and platelet-rich zone PRFM groups.
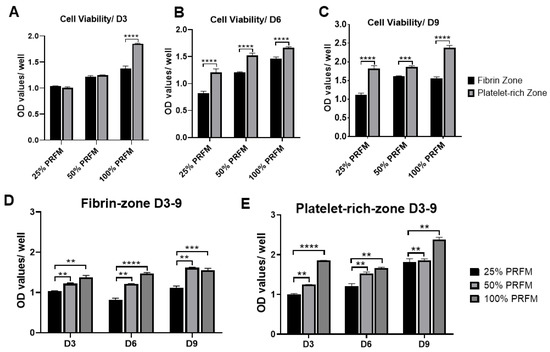
Figure 3. Differential effects of fibrin zone- and platelet-rich zone-platelet-rich fibrin conditioned medium (PRFM) on viability of tenocytes. (A–C) The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was used to assess the viability of tenocytes under fibrin zone- and platelet-rich zone PRFM treatments at three different time points. On day 6 and day 9, platelet-rich zone PRFM was more potent than fibrin zone PRFM in promoting cell growth, regardless of PRFM concentration. (D,E) Dose-dependent effects of fibrin zone- and platelet-rich zone PRFM on viability of tenocytes during a nine-day culture. The bars show the mean ± SD fold change (n = 4) of each group. D3, day3, D6, day 6, D9, day 9. * p < 0.05; ** p < 0.01; *** p < 0.001; *** p < 0.0001.
1.4. PRF Promotes Tenogenic Gene Expression
PRF Promotes Tenogenic Gene Expression
In this experiment, the PRFMs prepared from platelet-rich zone was used. In addition, the effects of different concentration of PRFM was compared with standard culture medium (DMEM/F-12 containing 10% FBS (10% FBSM)). The type I collagen (Col1a1) expression was upregulated from day 3 to day 6 in 50% and 100% PRFM groups (Figure 4A). No significant difference in type III collagen (Col3a1) expression was detected between 10% FBSM and PRFM groups (Figure 4B). As shown in Figure 4C,D, treatment of tenocytes with 100% PRFM significantly upregulated Tenomodulin (Tnmd) and Tenascin (Tnc) expressions. Compared with 10% FBSM, the 100% PRFM-treated tenocytes demonstrated 10-fold and 2.3-fold increases in Tnmd expression on days 3 and 6, respectively. On the other hand, the 50% and 100% PRFM group has 13.5-fold and 2.5-fold increase in Tnc expression compared with 10% FBSM after six-day cultivation. The results indicated that expression of tenogenic markers increased in the PRFM culture was correlated with PRFM concentration.
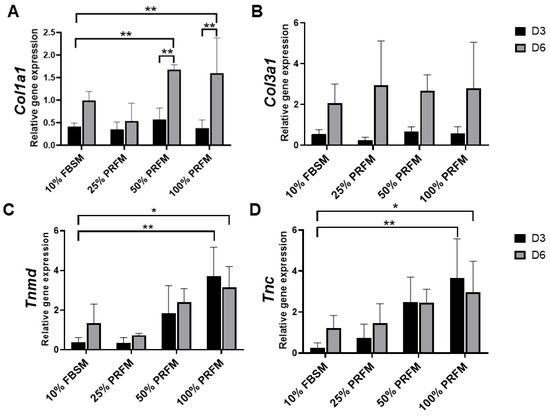
Figure 4. Quantitative expression profiles of (A) Collagen I (Col1a1), (B) Collagen III (Col3a1), (C) Tenomodulin (Tnmd), and (D) Tenascin (Tnc) of cultured tenocytes on day 3 and day 6. The asterisks indicate significant differences in the gene expression among all four groups: Dulbecco’s Modified Eagle’s Medium F-12 containing 10% fetal bovine serum (10% FBSM), and 25%, 50%, and 100% platelet-rich fibrin medium (PRFM) prepared from platelet-rich zone PRF. The bars show the mean ± SD fold change (n = 4) of each group. D3, day3, D6, day 6. * p< 0.05; ** p< 0.01.
1.5. Sonographic Findings
Sonographic Findings
The sonographic findings were evaluated based on the intratendinous morphology of the repaired tendon (Figure 5A–C). In the untreated group and heat-denatured PRF (dePRF) group, the longitudinal and transverse views showed that the defect region demonstrating inhomogeneous echo structure. Some anechoic areas were detected as discontinuous, fibrillary echo texture resulting from incomplete healing of tendon defects (Figure 5D–G). Moreover, some intratendinous hyperechoic areas which are suggestive of scar tissue formation or calcifications were also noted (Figure 5D–G). In the PRF group, the defect region demonstrating continuous fibrillary appearance with tendon fiber are well aligned along the long axis of the native tendon (Figure 5H,I).
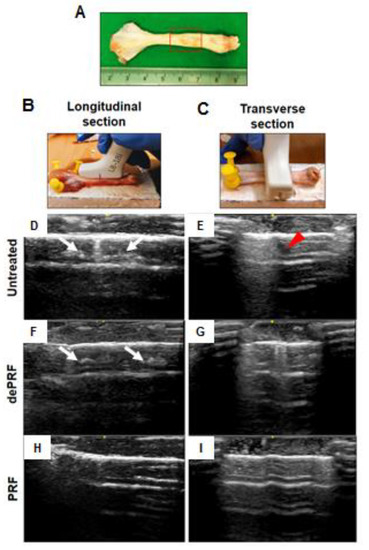
Figure 5. Sonographic evaluation of the healing process of rabbit Achilles tendon after platelet-rich fibrin (PRF) implantation. (A) Gross view of tendon specimens. The red rectangular frame highlighted the repaired zone. (B,C) The tendon specimens were evaluated longitudinally (B) and transversely (C). Tendon sonographic images in the untreated control (D,E), the heat-denatured PRF group (dePRF) (F,G), and the PRF group six weeks after treatment (H,I). The white arrows indicate intratendinous hyperechoic areas suggesting scar tissue formation. The red arrowhead indicates intratendinous fluid accumulation.
1.6. Histological Analysis of Tendon Healing
Histological Analysis of Tendon Healing
Figure 6A–D shows the histological images of the repaired tissues at three groups stained by hematoxylin and eosin (H&E) and Masson Trichrome (MT) at different magnification powers. In the untreated group, a cluster of round-shaped chondrocyte-like cells was found at the repaired zone. Moreover, the fibers were loosely composed without significant order (upper panel, Figure 6A–D). In dePRF group, the repaired zone was filled with heterogeneous tissues formed by fragmented collagen bundles. The tissues consist of cells in different shapes and sizes (middle panel, Figure 6A–D). The cartilage-like tissues and granulation tissues respective to untreated group and dePRF group were scarcely stained by MT. In the PRF group, most of the cells at the repaired zone were elongated in shape, resembling normal tenocytes. The cellularity is remarkably higher, and the collagen fibers were wavy and formed a crimping pattern (lower panel, Figure 6A–D). Moreover, the extracellular matrix composition at the repaired zone in the PRF group was strongly stained by MT, indicating dense collagen formation.
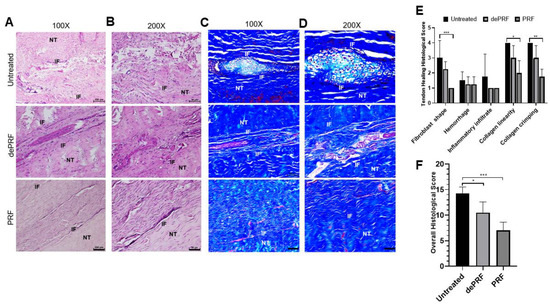
Figure 6. Histological evaluation of tendon healing after post-operative 6 weeks by hematoxylin and eosin (H&E) and Masson Trichrome (MT) staining. (A,B) The H&E staining of repaired tissue of untreated control (upper panel), heat-denatured PRF (dePRF) (middle panel), and PRF groups (lower panel). (C,D) Characterization of matrix components formed at the repaired zone by MT staining in the untreated control (upper panel), heat-denatured PRF (dePRF) (middle panel), and PRF groups (lower panel). (E,F) Histological scores of repaired tissue in three groups. NT, normal tendon; IF, interface. Scale bar: A, C: 100 μm; B, D: 50 μm. The bars show the mean ± SD (n = 3) of each group. * p< 0.05; ** p< 0.01; *** p < 0.001.
Quantitatively, the individual evaluation of five parameters revealed that in PRF group, the cells at the repaired zone were more fibroblast like cells resided in organized collagen fibers (Figure 6E). The overall histological score of dePRF (10.5 ± 2.6) and the PRF group (7 ± 1.6) were better than the untreated group (14.25 ± 1.26, p < 0.05) (Figure 6F). No significant difference in vascular hyperplasia and inflammatory infiltration were noted among the three groups.
2. Discussion
In the past few decades, platelet concentrate has emerged as an adjunct therapeutic for musculoskeletal injury. The rationale for its use is largely dependent on its functional components, which include a variety of growth factors, coagulation factors, adhesion molecules, cytokines, and chemokines [2][3][4][5][6][14,25,26,27,28]. Upon activation, the platelets can release these anabolic growth factors at concentrations significantly higher than the baseline blood levels [5][27].
The aims of this present study are to demonstrate the utility and capacity of PRF to serve as a “cytokine-delivery vehicle” in promoting Achilles tendon healing. In our previous studies, PRF demonstrated pronounced effects on cellular migration, viability, and differentiation both in vitro and in vivo, which may translate into a viable strategy in promoting tendinous healing. Nonetheless, the cytokine-release kinetics of PRF and its influence on tendinous healing remained largely unknown. Based on our results, the essence portion of PRF was successfully defined and its bioactivity on cultured tenocytes were revealed. When implanted in vivo, PRF can improve functional tendinous healing evidenced by imaging and histological results. From a clinical standpoint, our data demonstrate a new and effective way to improve tendinous healing.
Platelet-rich fibrin (PRF) is a second-generation platelet concentrate produced from autologous blood obtained immediately after single-spin centrifugation [2][14]. Rapid activation of the coagulation cascade and synthesis of thrombin take place when the platelets come into contact with the glass particles of the test tube. The ATP is co-packaged in platelet–dense granules with serotonin, Ca2+, and ADP and are secreted when the platelets are activated [1][7][24,29]. In the current study, the distribution of activated platelets on the PRF scaffold was identified by detecting the intensity of emitted bioluminescence light transients produced through the reaction of luciferin and activated platelet–released ATP. Geographically, we found that there were two distinct zones in the PRF scaffold in terms of bioluminescence intensity, known as the fibrin zone located at the upper half and the platelet-rich zone at the bottom half. Regarding PRF microstructure, some authors have revealed the inconsistency of scaffold compactness and porosities [4][8][26,30]. Therefore, we assume that the accumulation of activated platelets at the bottom half of PRF scaffold are attributed to the tighter compactness and smaller porosities of fibrin meshwork followed centrifugation. From functional perspective, the bottom-half platelet-rich zone was thus defined as the “PRF essence” region due to the relatively higher number of platelets. Despite the variations in PRF cytokine from batch to batch, the PRF essence consistently contains highly concentrated platelets and cytokines. When applied in vivo, we believe that the features of PRF essence may compensate for the individual discrepancy to facilitate tendinous healing.
Since platelets aggregate along the fibrin fibers during clotting, the resultant three-dimensional (3D) scaffold could act as a reservoir of growth factors. Moreover, the equilateral junctions of the PRF 3D matrix allow the establishment of a fine and flexible fibrin network that enable cytokines enmeshment [2][9][14]. Despite the increase in the clinical use of the platelet concentrates such as PRP for local tissue healing and regeneration, little is known about the biomolecule characteristic of these therapeutics in terms of cytokine-release kinetics. In this study, growth factors such as PDGF (AA and BB isoforms), FGF-2, TGF-β1, and IGF-1 were chosen for analysis because there are basic cytokines identified in platelets that play crucial role in cell proliferation, differentiation, and chemotaxis [9][5]. In this study, the time-sequential cytokine-release kinetics was revealed by determining the cytokine concentrations of PRF-immersed supernatant at different time points. It was demonstrated that most growth factors could be detected at the first hour. Interestingly, an increasing trend of PDGF-AA and BB, and FGF-2 release was detected even after six days. The results of this study were consistent with previous reports, suggesting that the PRF tightly packed fibrin fibers can exhibit a locking effect on the cytokines [8][10][30,31]. The results of this study also lend support to our hypothesis that PRF could serve as a cytokine-delivery vehicle that would sustainably deliver bioactive molecules in promoting tendinous healing.
The functional healing of tendinous injury relied on the formation of tendinous tissue composed of sufficient cells with tenogenic potential. In this study, we found that PRF-conditioned medium (PRFM) could stimulate tenocytes proliferation and tenogenic matrix production. Based on the cytokine-release kinetic analysis, we found that the increased anabolic activities of tenocytes may be attributed to the cytokines originating from PRF. PDGF is a powerful mitogen for fibroblast which could increase cell density and proliferation [11][32]. FGF could increase production of extracellular matrix while applied in vivo [12][33]. When IGF-1 and TGF-β were delivered to the repair site of supraspinatus tendon-to-bone insertions of rats, increased cell proliferation, vascularity, and the production of fibrous repair tissue could be observed [13][34]. Our results showed that the number of viable tenocytes treated with proportionally increased concentrations of PRFM (25%, 50%, and 100%) increased in a dose-dependent manner during the nine-day culture (Figure 3). On the other hand, the expression of tendon-related genes such as Tnmd and Tnc were significantly upregulated after treatment with high-concentration PRFM. Tnmd is a gene that is highly expressed in tendons and is required for tenocytes proliferation and tendon maturation [14][15][16][35,36,37]. Tnc is a glycoprotein that plays an important role for tenocytes adaptation to compression [17][38]. Moreover, PRFM has comparable effects as 10% FBSM on Col1a1 and Col3a1 gene expressions. Both types of collagen are important molecules in the tendon extracellular matrix and are produced in high quantity at the early phase of tendinous healing [18][19][20][2,3,39]. Collectively, the in vitro results indicated that PRF exerts anabolic effects on tenocytes proliferation and tenogenic differentiation, which would benefit for in vivo tendinous healing.
PRF is a biomaterial yielded by a natural polymerization process during centrifugation [2][14]. Its natural fibrin architecture is beneficial for the containment and slow release of growth factors over time [8][21][13,30]. When implanted in vivo, the PRF scaffold could serve as a delivery platform for growth factors and provides an architecture for the attachment, proliferation, and migration of cells at the target site. The in vivo results demonstrated superior tendinous healing in the PRF-implanted group compared to control and the dePRF-implanted group, evidenced by sonographic and histological findings. For sonographic analysis, the intratendinous hyperechoic areas found in the control and dePRF groups may be ascribed to scar tissues comprised of different types of cells and an unorganized matrix. In contrast, the intratendinous morphology of the repaired tendon in PRF are mostly isoechoic. Moreover, the repaired tissue displayed a fibrillary appearance. These imaging findings indicate that the collagen fibers of the repaired tissue are cross linked and align along the long axis of the tendon, suggesting that the remodeling phase was underway.
In general, tendon healing progresses through an inflammatory phase (days), followed by a reparative phase (weeks), and ended with a remodeling phase (months) [20][22][4,39]. Our histological results showed that PRF implantation leads to the initiation of a tendon reparative process characterized by increased tenocytes proliferation and differentiation. Despite the relatively high number of proliferative tenocytes found at the healing zone, most of the cells are spindly and elongated in shape. In addition, the collagen fibers were continuous, wavy, and aligned longitudinally in one direction. On the other hand, in control and dePRF rabbits, most of the tenocytes are oval/round in shape, resembling chondrocytes-like cells with basophilic lacanue. It was reported that the presence of cartilage is a reliable indicator of an inferior repair process and poorer biomechanics [23][40]. Moreover, the fiber arrangements at the healing zone was loosed, disorganized, and fragmented without an identifiable pattern. Taken together, it was reasonably to propose that PRF could improve the reparative process by upregulating the tenocytes growth and collagen production. However, the histological findings also suggest that the remodeling process of tendinous injury is yet to be completed in terms of increased cell number and immature matrix synthesis.
Our data showed the potential of PRF in promoting Achilles tendon healing in a preclinical trial with an animal model. However, our study has several limitations. First, this preliminary study was conducted to evaluate the biological role of PRF in tendinous healing process at six weeks postoperatively. However, it might be more thorough to assess the surgical repair outcomes over different time points. The results also indicate that a longer study period may be needed to evaluate the complete course of tendinous healing.
